Abstract
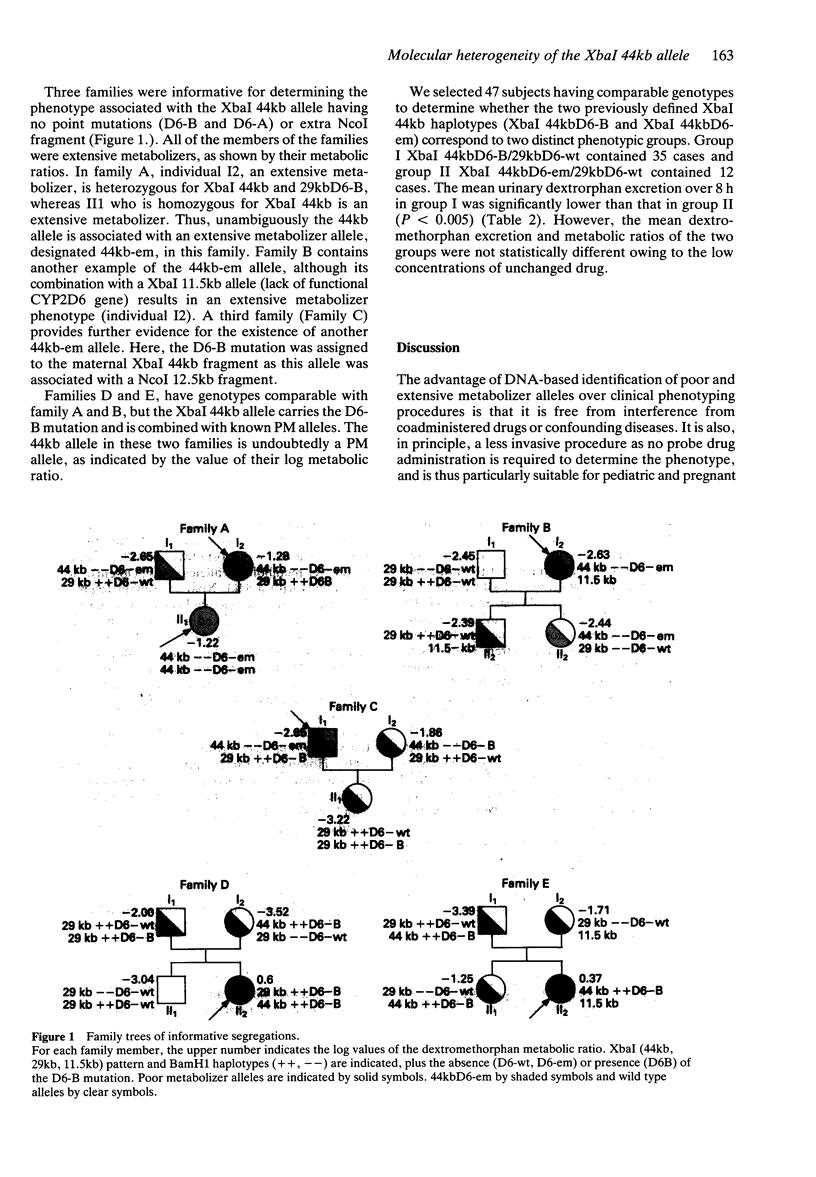
1. Cytochrome P450 debrisoquine (CYP2D6) activity is polymorphic and under genetic control. Most Caucasians are extensive metabolizers, but 5%-10% are poor metabolizers. 2. Restriction fragment length polymorphism analysis of the CYP2D6 locus identifies a 29kb XbaI fragment, either normal (D6-wt) or mutated, and three mutated XbaI alleles (44kb, 11.5kb and 16 + 9kb). The 44kb allele was initially considered as a poor metabolizer allele owing to a D6-B mutation, but cases of 44kb allele not carrying the D6-B, and therefore potentially functional, have been found. The degree of molecular heterogeneity of this allele was investigated by phenotype and genotype analysis of families. 3. Thirty-one French Caucasian families, representing 117 individuals, possessing at least one 44kb allele in each family were selected. Phenotypes were determined using dextromethorphan, and the XbaI, NcoI and BamH1 RFLPs of 42 independent chromosomes were analyzed. 4. 80% of the XbaI 44kb alleles carried the CYP2D6-B mutation and had an additional NcoI fragment (12.5kb or 4.8kb). The remaining 20% did not carry the CYP2D6-B or A mutations and had no extra NcoI fragment. 5. Information on three families demonstrated that 44kb alleles not carrying the CYP2D6-B mutation were associated with the extensive metabolizer phenotype. 6. We conclude that a substantial percentage of XbaI 44kb alleles is associated with a functional CYP2D gene, and therefore, that the XbaI 44kb allele is not consistently a poor metaboliser allele.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alván G., Bechtel P., Iselius L., Gundert-Remy U. Hydroxylation polymorphisms of debrisoquine and mephenytoin in European populations. Eur J Clin Pharmacol. 1990;39(6):533–537. doi: 10.1007/BF00316090. [DOI] [PubMed] [Google Scholar]
- Broly F., Gaedigk A., Heim M., Eichelbaum M., Morike K., Meyer U. A. Debrisoquine/sparteine hydroxylation genotype and phenotype: analysis of common mutations and alleles of CYP2D6 in a European population. DNA Cell Biol. 1991 Oct;10(8):545–558. doi: 10.1089/dna.1991.10.545. [DOI] [PubMed] [Google Scholar]
- Brosen K. Recent developments in hepatic drug oxidation. Implications for clinical pharmacokinetics. Clin Pharmacokinet. 1990 Mar;18(3):220–239. doi: 10.2165/00003088-199018030-00004. [DOI] [PubMed] [Google Scholar]
- Brøsen K., Gram L. F. Clinical significance of the sparteine/debrisoquine oxidation polymorphism. Eur J Clin Pharmacol. 1989;36(6):537–547. doi: 10.1007/BF00637732. [DOI] [PubMed] [Google Scholar]
- Dahl M. L., Johansson I., Palmertz M. P., Ingelman-Sundberg M., Sjöqvist F. Analysis of the CYP2D6 gene in relation to debrisoquin and desipramine hydroxylation in a Swedish population. Clin Pharmacol Ther. 1992 Jan;51(1):12–17. doi: 10.1038/clpt.1992.2. [DOI] [PubMed] [Google Scholar]
- Daly A. K., Armstrong M., Idle J. R. Molecular genotyping to predict debrisoquine hydroxylation phenotype. Lancet. 1990 Oct 6;336(8719):889–890. doi: 10.1016/0140-6736(90)92413-c. [DOI] [PubMed] [Google Scholar]
- Eichelbaum M., Gross A. S. The genetic polymorphism of debrisoquine/sparteine metabolism--clinical aspects. Pharmacol Ther. 1990;46(3):377–394. doi: 10.1016/0163-7258(90)90025-w. [DOI] [PubMed] [Google Scholar]
- Evans D. A., Mahgoub A., Sloan T. P., Idle J. R., Smith R. L. A family and population study of the genetic polymorphism of debrisoquine oxidation in a white British population. J Med Genet. 1980 Apr;17(2):102–105. doi: 10.1136/jmg.17.2.102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans W. E., Relling M. V. Xbal 16- plus 9-kilobase DNA restriction fragments identify a mutant allele for debrisoquin hydroxylase: report of a family study. Mol Pharmacol. 1990 May;37(5):639–642. [PubMed] [Google Scholar]
- Gaedigk A., Blum M., Gaedigk R., Eichelbaum M., Meyer U. A. Deletion of the entire cytochrome P450 CYP2D6 gene as a cause of impaired drug metabolism in poor metabolizers of the debrisoquine/sparteine polymorphism. Am J Hum Genet. 1991 May;48(5):943–950. [PMC free article] [PubMed] [Google Scholar]
- Gonzalez F. J., Meyer U. A. Molecular genetics of the debrisoquin-sparteine polymorphism. Clin Pharmacol Ther. 1991 Sep;50(3):233–238. doi: 10.1038/clpt.1991.131. [DOI] [PubMed] [Google Scholar]
- Gough A. C., Miles J. S., Spurr N. K., Moss J. E., Gaedigk A., Eichelbaum M., Wolf C. R. Identification of the primary gene defect at the cytochrome P450 CYP2D locus. Nature. 1990 Oct 25;347(6295):773–776. doi: 10.1038/347773a0. [DOI] [PubMed] [Google Scholar]
- Hanioka N., Kimura S., Meyer U. A., Gonzalez F. J. The human CYP2D locus associated with a common genetic defect in drug oxidation: a G1934----A base change in intron 3 of a mutant CYP2D6 allele results in an aberrant 3' splice recognition site. Am J Hum Genet. 1990 Dec;47(6):994–1001. [PMC free article] [PubMed] [Google Scholar]
- Jacqz E., Dulac H., Mathieu H. Phenotyping polymorphic drug metabolism in the French Caucasian population. Eur J Clin Pharmacol. 1988;35(2):167–171. doi: 10.1007/BF00609247. [DOI] [PubMed] [Google Scholar]
- Johansson I., Yue Q. Y., Dahl M. L., Heim M., Säwe J., Bertilsson L., Meyer U. A., Sjöqvist F., Ingelman-Sundberg M. Genetic analysis of the interethnic difference between Chinese and Caucasians in the polymorphic metabolism of debrisoquine and codeine. Eur J Clin Pharmacol. 1991;40(6):553–556. doi: 10.1007/BF00279968. [DOI] [PubMed] [Google Scholar]
- Kagimoto M., Heim M., Kagimoto K., Zeugin T., Meyer U. A. Multiple mutations of the human cytochrome P450IID6 gene (CYP2D6) in poor metabolizers of debrisoquine. Study of the functional significance of individual mutations by expression of chimeric genes. J Biol Chem. 1990 Oct 5;265(28):17209–17214. [PubMed] [Google Scholar]
- Kimura S., Umeno M., Skoda R. C., Meyer U. A., Gonzalez F. J. The human debrisoquine 4-hydroxylase (CYP2D) locus: sequence and identification of the polymorphic CYP2D6 gene, a related gene, and a pseudogene. Am J Hum Genet. 1989 Dec;45(6):889–904. [PMC free article] [PubMed] [Google Scholar]
- Mahgoub A., Idle J. R., Dring L. G., Lancaster R., Smith R. L. Polymorphic hydroxylation of Debrisoquine in man. Lancet. 1977 Sep 17;2(8038):584–586. doi: 10.1016/s0140-6736(77)91430-1. [DOI] [PubMed] [Google Scholar]
- Meyer U. A., Skoda R. C., Zanger U. M. The genetic polymorphism of debrisoquine/sparteine metabolism-molecular mechanisms. Pharmacol Ther. 1990;46(2):297–308. doi: 10.1016/0163-7258(90)90096-k. [DOI] [PubMed] [Google Scholar]
- Motassim N., Decolin D., Le Dinh T., Nicolas A., Siest G. Direct determination of dextromethorphan and its three metabolites in urine by high-performance liquid chromatography using a precolumn switching system for sample clean-up. J Chromatogr. 1987 Nov 27;422:340–345. doi: 10.1016/0378-4347(87)80473-5. [DOI] [PubMed] [Google Scholar]
- Mura C., Broyard J. P., Jacqz-Aigrain E., Krishnamoorthy R. Distinct phenotypes and genotypes of debrisoquine hydroxylation among Europeans and Chinese. Br J Clin Pharmacol. 1991 Jul;32(1):135–136. doi: 10.1111/j.1365-2125.1991.tb05629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nebert D. W., Gonzalez F. J. P450 genes: structure, evolution, and regulation. Annu Rev Biochem. 1987;56:945–993. doi: 10.1146/annurev.bi.56.070187.004501. [DOI] [PubMed] [Google Scholar]
- Skoda R. C., Gonzalez F. J., Demierre A., Meyer U. A. Two mutant alleles of the human cytochrome P-450db1 gene (P450C2D1) associated with genetically deficient metabolism of debrisoquine and other drugs. Proc Natl Acad Sci U S A. 1988 Jul;85(14):5240–5243. doi: 10.1073/pnas.85.14.5240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sloan T. P., Lancaster R., Shah R. R., Idle J. R., Smith R. L. Genetically determined oxidation capacity and the disposition of debrisoquine. Br J Clin Pharmacol. 1983 Apr;15(4):443–450. doi: 10.1111/j.1365-2125.1983.tb01528.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turgeon J., Evans W. E., Relling M. V., Wilkinson G. R., Roden D. M. Phenotypic debrisoquine 4-hydroxylase activity among extensive metabolizers is unrelated to genotype as determined by the Xba-I restriction fragment length polymorphism. Br J Clin Pharmacol. 1991 Sep;32(3):283–288. doi: 10.1111/j.1365-2125.1991.tb03900.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf C. R., Moss J. E., Miles J. S., Gough A. C., Spurr N. K. Detection of debrisoquine hydroxylation phenotypes. Lancet. 1990 Dec 8;336(8728):1452–1453. doi: 10.1016/0140-6736(90)93163-j. [DOI] [PubMed] [Google Scholar]


