Abstract
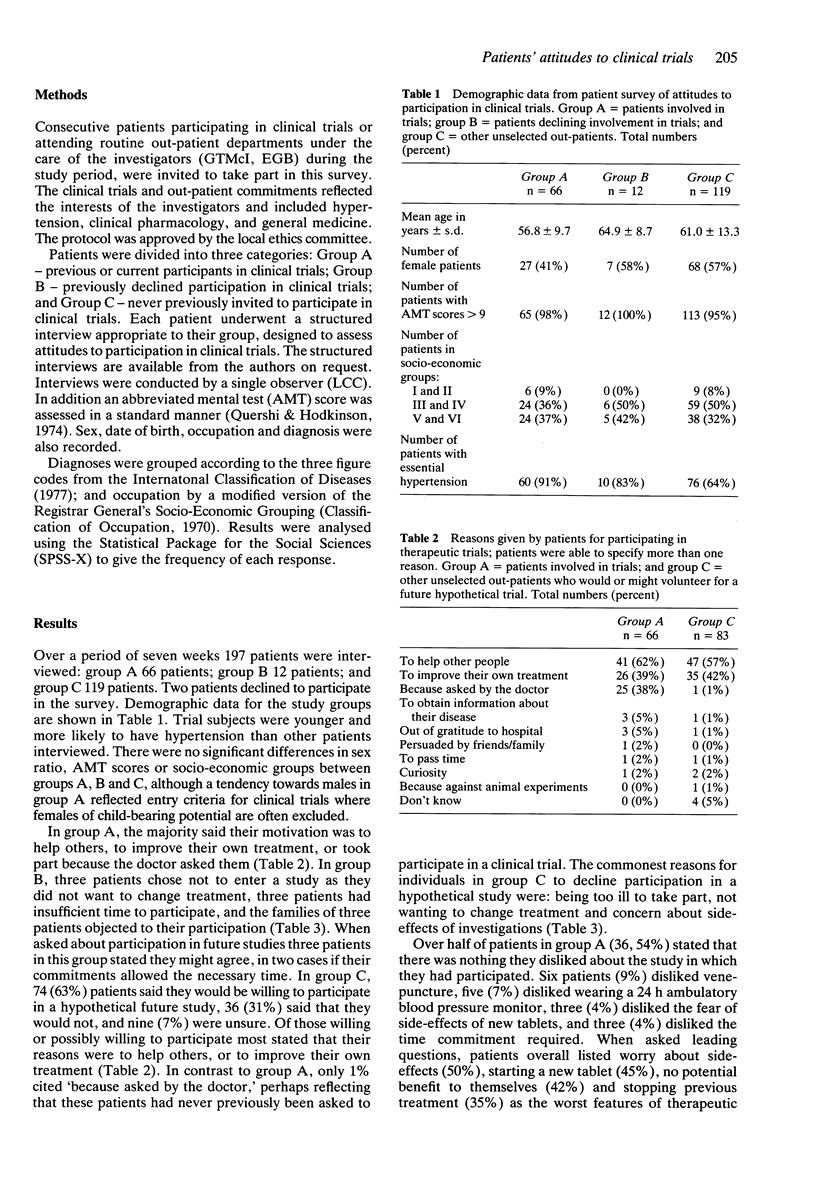
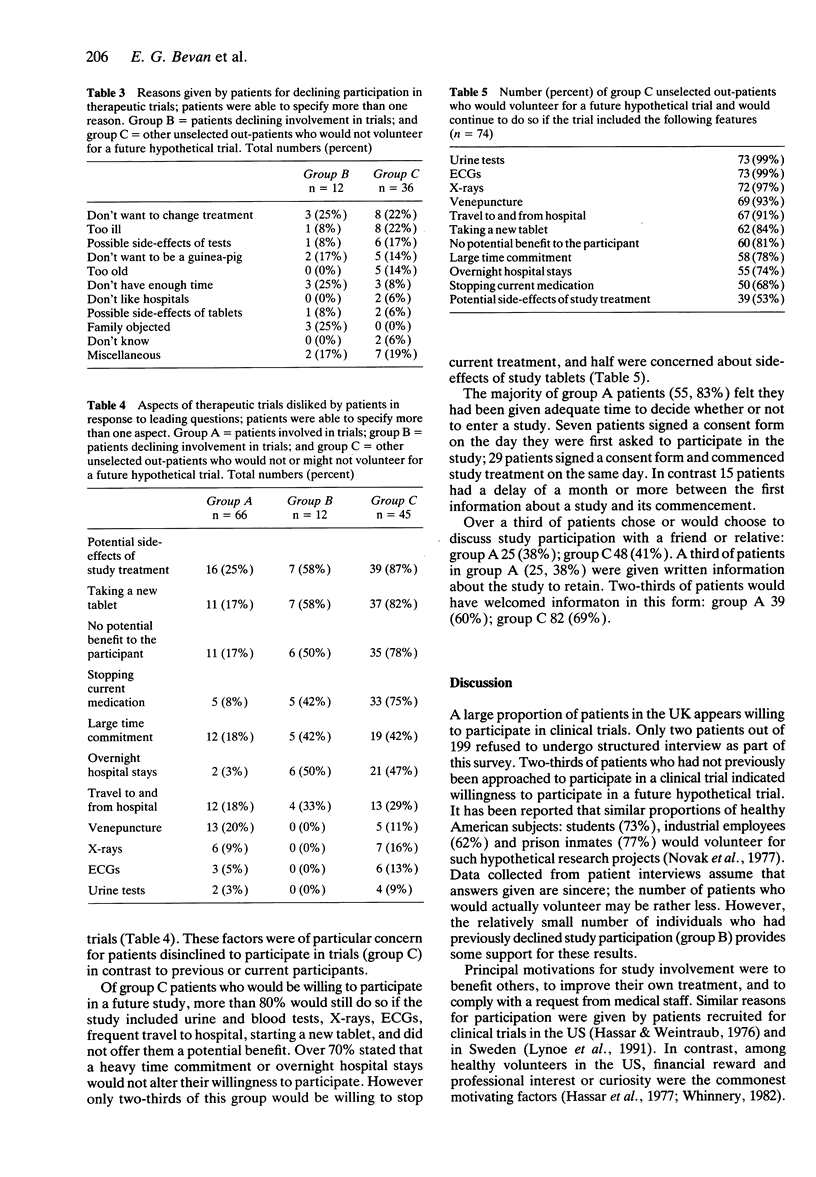
1. In order to assess attitudes of patients to participation in therapeutic trials 197 patients underwent structured interviews conducted by a single observer. Of these patients 66 were previous or current participants in clinical trials (group A), 12 had declined participation in a trial (group B), and 119 had never been invited to participate in research (group C). 2. In group A, 62% stated their motivation for participation was to help others and 39% to improve their own treatment, but in 38% participation was to comply with the doctor's request. Two-thirds of group C patients would or might participate in a future hypothetical trial; of these more than half (57%) would do so to help others, and 42% to improve their own treatment. 3. Of the 12 patients who had declined entry into a study (group B) three did not want to alter their current therapy, three had insufficient time to participate in the particular trial, and in three cases relatives objected to their participation. Group C patients who stated they would not participate in trials gave being too ill (22%), not wanting to change treatment (22%), and fear of side-effects (17%) as the commonest reasons for declining. 4. In group A, 83% felt they had adequate time to consider their participation. Nearly two-thirds of patients (60%) would have liked written information to retain for reference, whereas only 38% were provided with information in this form. Over half of these patients (54%) disliked no aspect of the study in which they participated. Venepuncture and other uncomfortable procedures were least popular.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Hassar M., Pocelinko R., Weintraub M., Nelson D., Thomas G., Lasagna L. Free-living volunteer's motivations and attitudes toward pharmacologic studies in man. Clin Pharmacol Ther. 1977 May;21(5):515–519. doi: 10.1002/cpt1977215515. [DOI] [PubMed] [Google Scholar]
- Hassar M., Weintraub M. "Uniformed" consent and the wealthy volunteer: an analysis of patient volunteers in a clinical trial of a new anti-inflammatory drug. Clin Pharmacol Ther. 1976 Oct;20(4):379–386. doi: 10.1002/cpt1976204379. [DOI] [PubMed] [Google Scholar]
- Lynöe N., Sandlund M., Dahlqvist G., Jacobsson L. Informed consent: study of quality of information given to participants in a clinical trial. BMJ. 1991 Sep 14;303(6803):610–613. doi: 10.1136/bmj.303.6803.610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak Ervin, Seckman Clarence E., Stewart Raymond D. Motivations for volunteering as research subjects. J Clin Pharmacol New Drugs. 1977 Jul;17(7):365–371. [PubMed] [Google Scholar]
- Qureshi K. N., Hodkinson H. M. Evaluation of a ten-question mental test in the institutionalized elderly. Age Ageing. 1974 Aug;3(3):152–157. doi: 10.1093/ageing/3.3.152. [DOI] [PubMed] [Google Scholar]
- Whinnery J. E. Motivational analysis of human volunteers for centrifuge acceleration research. Aviat Space Environ Med. 1982 Oct;53(10):1017–1020. [PubMed] [Google Scholar]


