Abstract
1. Stride length is highly relevant to mobility and is sensitive to the effects of levodopa in Parkinsonism. Its selection as the primary outcome criterion allowed comparison of two levodopa/decarboxylase inhibitor formulations using a small number of subjects. 2. It is also desirable to improve stability. An instrumental method, based on infrared telemetry, has been developed which obtains both distance/time measures of gait and broadness of base, as measured by foot separation at mid-swing. The latter was used as a subsidiary outcome criterion. 3. Nine patients (aged 57 to 77 years) then receiving maintenance therapy for idiopathic Parkinsonism with Sinemet CR alone, but who had previously experienced end of dose effect within 4 h of receiving a dose of a conventional formulation of levodopa/decarboxylase inhibitor, were studied. 4. They received, in random order and at least 4 days apart, single doses of one tablet of Sinemet CR (200 mg levodopa/50 mg carbidopa) and of two capsules of Madopar CR (each 100 mg levodopa/25 mg benserazide), with placebo balance, at 10.00 h. Gait analysis was carried out immediately before and half-hourly for 7 h after a challenge. No routine doses of Sinemet CR were taken between 22.00 h on the night before and 17.00 h on the day of a challenge. 5. Analysis of variance showed a highly significant difference in mean stride length (P < 0.001) and in mean foot separation (P = 0.01) between serial time points, irrespective of the nature of treatment. There appeared to be a useful therapeutic response to both challenges.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF

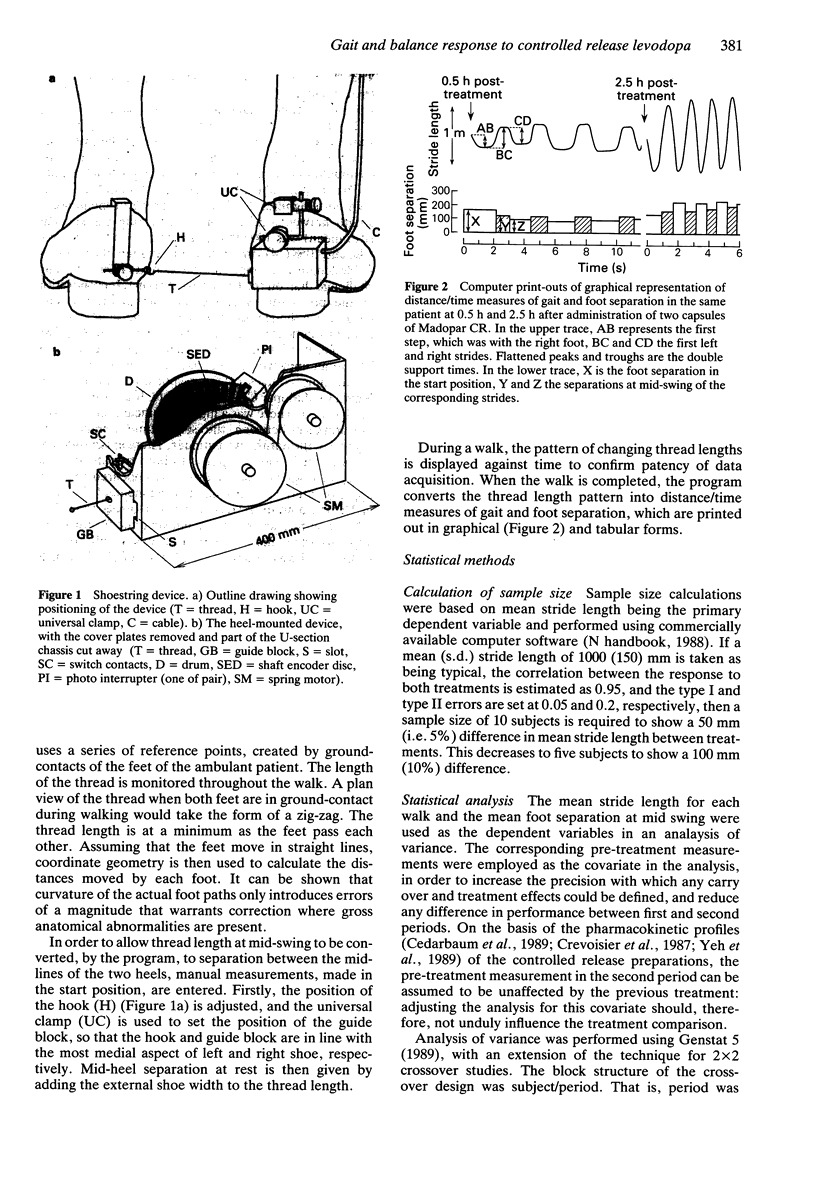

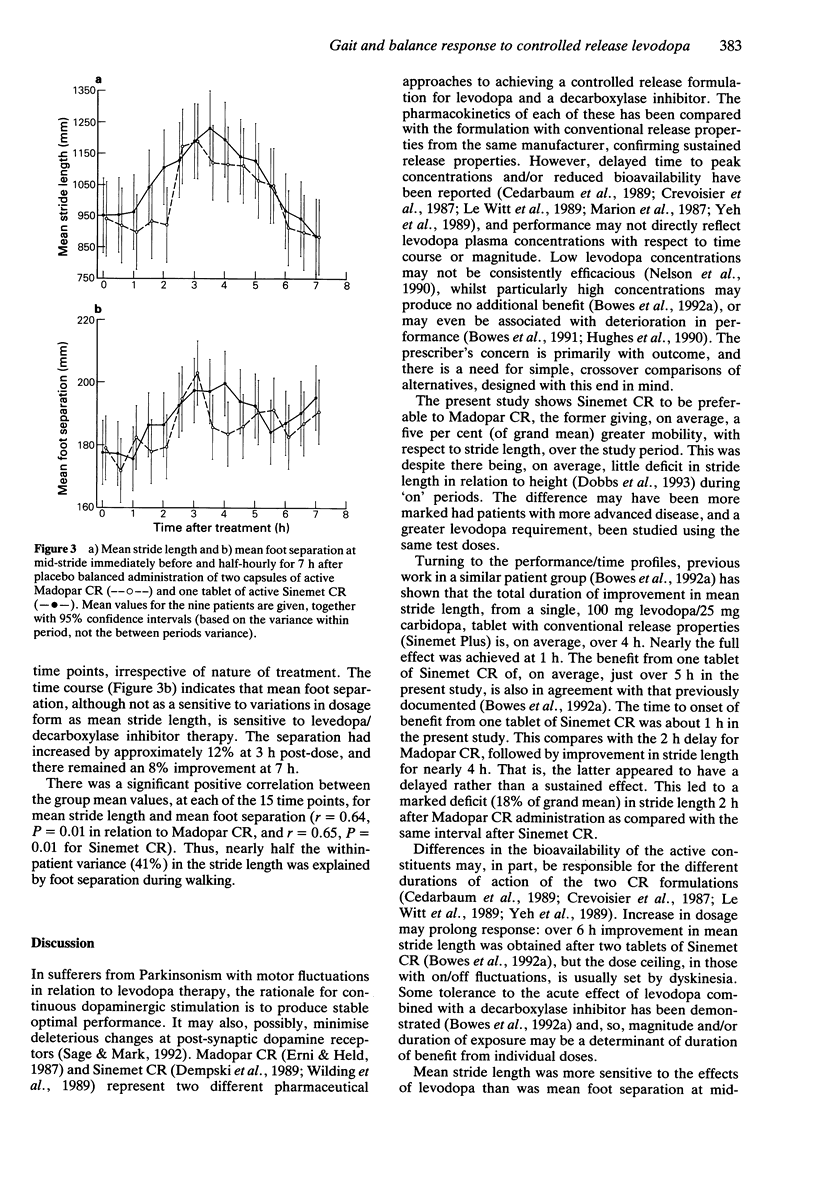


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bowes S. G., Clark P. K., Charlett A., O'Neill C. J., Leeman A. L., Weller C., Nicholson P. W., Deshmukh A. A., Dobbs S. M., Dobbs R. J. Objective outcome criteria in trials of anti-parkinsonian therapy in the elderly: sensitivity, specificity and reliability of measures of brady- and hypo-kinesia. Br J Clin Pharmacol. 1991 Mar;31(3):295–304. doi: 10.1111/j.1365-2125.1991.tb05533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowes S. G., Dobbs R. J., Henley M., Charlett A., O'Neill C. J., Nicholson P. W., Purkiss A. G., Weller C., Dobbs S. M. Objective evidence for tolerance, against a background of improvement, during maintenance therapy with controlled release levodopa/carbidopa. Eur J Clin Pharmacol. 1992;43(5):483–489. doi: 10.1007/BF02285089. [DOI] [PubMed] [Google Scholar]
- Cedarbaum J. M., Kutt H., McDowell F. H. A pharmacokinetic and pharmacodynamic comparison of Sinemet CR (50/200) and standard Sinemet (25/100). Neurology. 1989 Nov;39(11 Suppl 2):38–59. [PubMed] [Google Scholar]
- Crevoisier C., Hoevels B., Zürcher G., Da Prada M. Bioavailability of L-dopa after Madopar HBS administration in healthy volunteers. Eur Neurol. 1987;27 (Suppl 1):36–46. doi: 10.1159/000116173. [DOI] [PubMed] [Google Scholar]
- Dempski R. E., Scholtz E. C., Oberholtzer E. R., Yeh K. C. Pharmaceutical design and development of a Sinemet controlled-release formulation. Neurology. 1989 Nov;39(11 Suppl 2):20–24. [PubMed] [Google Scholar]
- Dobbs R. J., Charlett A., Bowes S. G., O'Neill C. J., Weller C., Hughes J., Dobbs S. M. Is this walk normal? Age Ageing. 1993 Jan;22(1):27–30. doi: 10.1093/ageing/22.1.27. [DOI] [PubMed] [Google Scholar]
- Erni W., Held K. The hydrodynamically balanced system: a novel principle of controlled drug release. Eur Neurol. 1987;27 (Suppl 1):21–27. doi: 10.1159/000116171. [DOI] [PubMed] [Google Scholar]
- Hughes J. R., Bowes S. G., Leeman A. L., O'Neill C. J., Deshmukh A. A., Nicholson P. W., Dobbs S. M., Dobbs R. J. Parkinsonian abnormality of foot strike: a phenomenon of ageing and/or one responsive to levodopa therapy? Br J Clin Pharmacol. 1990 Feb;29(2):179–186. doi: 10.1111/j.1365-2125.1990.tb03617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirollos C., O'Neill C. J., Dobbs R. J., Charlett A., Bowes S. G., Weller C., Purkiss A. G., Hunt W. B., Dobbs S. M. Quantification of the cardinal signs of parkinsonism and of associated disability in spouses of sufferers. Age Ageing. 1993 Jan;22(1):20–26. doi: 10.1093/ageing/22.1.20. [DOI] [PubMed] [Google Scholar]
- Marion M. H., Stocchi F., Malcolm S. L., Quinn N. P., Jenner P., Marsden C. D. Single-dose studies of a slow-release preparation of levodopa and benserazide (Madopar HBS) in Parkinson's disease. Eur Neurol. 1987;27 (Suppl 1):54–58. doi: 10.1159/000116193. [DOI] [PubMed] [Google Scholar]
- Nelson M. V., Berchou R. C., LeWitt P. A., Kareti D., Galloway M. P. Pharmacodynamic modeling of concentration-effect relationships after controlled-release carbidopa/levodopa (Sinemet CR4) in Parkinson's disease. Neurology. 1990 Jan;40(1):70–74. doi: 10.1212/wnl.40.1.70. [DOI] [PubMed] [Google Scholar]
- Sage J. I., Mark M. H. The rationale for continuous dopaminergic stimulation in patients with Parkinson's disease. Neurology. 1992 Jan;42(1 Suppl 1):23–60. [PubMed] [Google Scholar]
- Weller C., Humphrey S. J., Kirollos C., Bowes S. G., Charlett A., Dobbs S. M., Dobbs R. J. Gait on a shoestring: falls and foot separation in parkinsonism. Age Ageing. 1992 Jul;21(4):242–244. doi: 10.1093/ageing/21.4.242. [DOI] [PubMed] [Google Scholar]
- Wilding I. R., Davis S. S., Melia C. D., Hardy J. G., Evans D. F., Short A. H., Sparrow R. A. Gastrointestinal transit of Sinemet CR in healthy volunteers. Neurology. 1989 Nov;39(11 Suppl 2):53–58. [PubMed] [Google Scholar]
- Yeh K. C., August T. F., Bush D. F., Lasseter K. C., Musson D. G., Schwartz S., Smith M. E., Titus D. C. Pharmacokinetics and bioavailability of Sinemet CR: a summary of human studies. Neurology. 1989 Nov;39(11 Suppl 2):25–38. [PubMed] [Google Scholar]


