Abstract
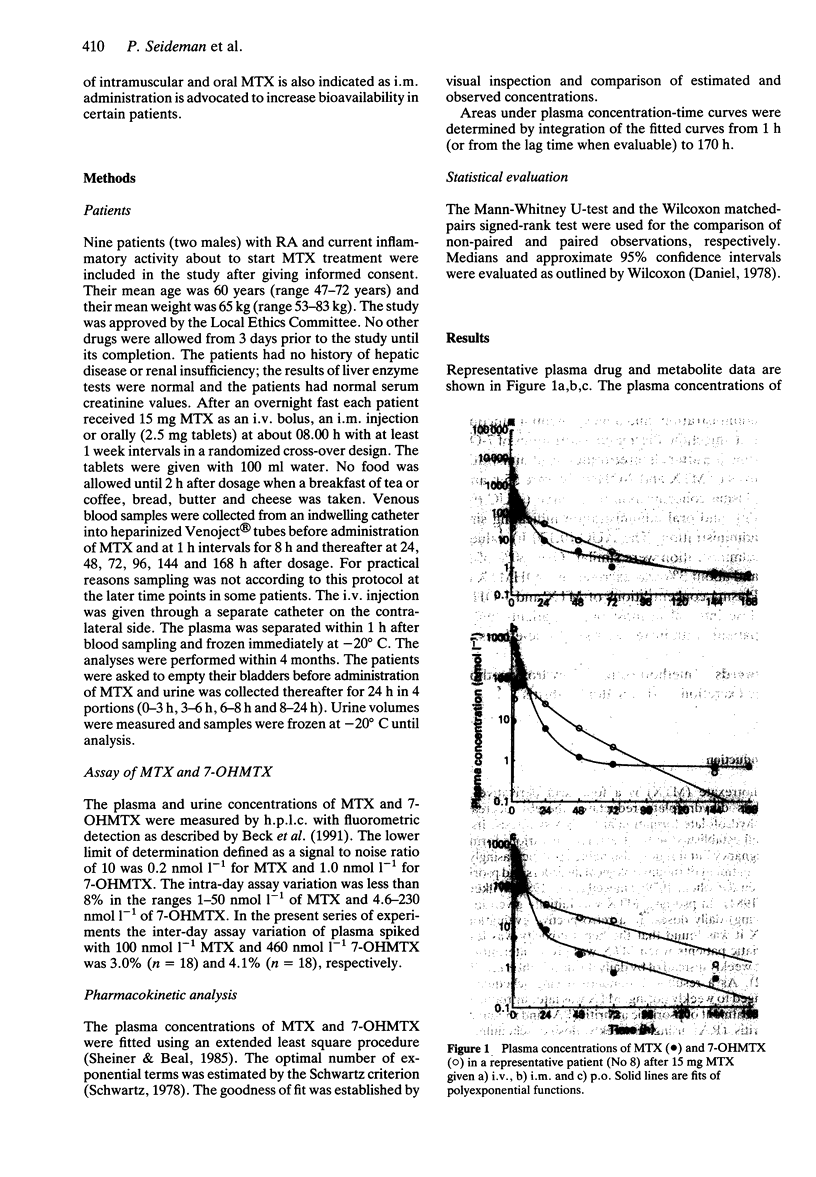
1. The pharmacokinetics of MTX and its 7-hydroxy metabolite (7-OHMTX) were investigated in nine patients with rheumatoid arthritis (RA). Each patient received 15 mg MTX i.v., i.m. and p.o. after an overnight fast in a randomized cross-over design. The plasma concentrations of MTX and 7-OHMTX were measured over 7 days and their urinary excretion over 24 h. 2. Plasma concentrations of MTX were described by a triexponential function after i.v. administration, a triexponential function with zero or first order absorption after oral administration, and a biexponential function with zero of first order absorption after i.m. injection. Plasma concentrations of 7-OHMTX were described by a biexponential function after all three routes of administration. The median terminal elimination half-lives of MTX and 7-OHMTX were 55 h and 116 h, respectively. The area under the plasma concentration-time curve (AUC (0,170 h)) of MTX did not differ between i.m. and oral administration indicating similar bioavailability after these routes of administration. The AUC (0,170 h) values of 7-OHMTX after i.v., oral and i.m. administration were similar. Over 80% of MTX was excreted in urine as intact drug and about 3% was excreted as 7-OHMTX during 24 h after drug administration. 3. Plasma concentrations of MTX and 7-OHMTX were measurable at the end of the dose interval in most of the patients and may help to identify non-responders or patients with increased risk of side-effects.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Balis F. M., Savitch J. L., Bleyer W. A. Pharmacokinetics of oral methotrexate in children. Cancer Res. 1983 May;43(5):2342–2345. [PubMed] [Google Scholar]
- Beck O., Seideman P., Wennberg M., Peterson C. Trace analysis of methotrexate and 7-hydroxymethotrexate in human plasma and urine by a novel high-performance liquid chromatographic method. Ther Drug Monit. 1991 Nov;13(6):528–532. doi: 10.1097/00007691-199111000-00011. [DOI] [PubMed] [Google Scholar]
- Christophidis N., Vajda F. J., Lucas I., Moon W. J., Louis W. J. Comparison of intravenous and oral high-dose methotrexate in treatment of solid tumours. Br Med J. 1979 Feb 3;1(6159):298–300. doi: 10.1136/bmj.1.6159.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl M. G., Gregory M. M., Scheuer P. J. Liver damage due to methotrexate in patients with psoriasis. Br Med J. 1971 Mar 20;1(5750):625–630. doi: 10.1136/bmj.1.5750.625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl M. G., Gregory M. M., Scheuer P. J. Methotrexate hepatotoxicity in psoriasis--comparison of different dose regimens. Br Med J. 1972 Mar 11;1(5801):654–656. doi: 10.1136/bmj.1.5801.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edelman J., Biggs D. F., Jamali F., Russell A. S. Low-dose methotrexate kinetics in arthritis. Clin Pharmacol Ther. 1984 Mar;35(3):382–386. doi: 10.1038/clpt.1984.47. [DOI] [PubMed] [Google Scholar]
- Evans W. E., Stewart C. F., Hutson P. R., Cairnes D. A., Bowman W. P., Yee G. C., Crom W. R. Disposition of intermediate-dose methotrexate in children with acute lymphocytic leukemia. Drug Intell Clin Pharm. 1982 Nov;16(11):839–842. doi: 10.1177/106002808201601105. [DOI] [PubMed] [Google Scholar]
- Herman R. A., Veng-Pedersen P., Hoffman J., Koehnke R., Furst D. E. Pharmacokinetics of low-dose methotrexate in rheumatoid arthritis patients. J Pharm Sci. 1989 Feb;78(2):165–171. doi: 10.1002/jps.2600780219. [DOI] [PubMed] [Google Scholar]
- Owen E. T., Cohen M. L. Methotrexate in Reiter's disease. Ann Rheum Dis. 1979 Feb;38(1):48–50. doi: 10.1136/ard.38.1.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson A. D., Mills S., Amineddine H. A., Long D. R., Craft A. W., Chessells J. M. Pharmacokinetics of oral and intramuscular methotrexate in children with acute lymphoblastic leukaemia. Cancer Chemother Pharmacol. 1987;20(3):243–247. doi: 10.1007/BF00570494. [DOI] [PubMed] [Google Scholar]
- Schrøder H., Clausen N., Ostergaard E., Pressler T. Pharmacokinetics of erythrocyte methotrexate in children with acute lymphoblastic leukemia during maintenance treatment. Cancer Chemother Pharmacol. 1986;16(2):190–193. doi: 10.1007/BF00256175. [DOI] [PubMed] [Google Scholar]
- Schrøder H., Fogh K. Methotrexate and its polyglutamate derivatives in erythrocytes during and after weekly low-dose oral methotrexate therapy of children with acute lymphoblastic leukemia. Cancer Chemother Pharmacol. 1988;21(2):145–149. doi: 10.1007/BF00257362. [DOI] [PubMed] [Google Scholar]
- Schrøder H. In vivo methotrexate kinetics and metabolism in human hematopoietic cells. Clinical significance of methotrexate concentrations in erythrocytes. Dan Med Bull. 1990 Feb;37(1):22–40. [PubMed] [Google Scholar]
- Sheiner L. B., Beal S. L. Pharmacokinetic parameter estimates from several least squares procedures: superiority of extended least squares. J Pharmacokinet Biopharm. 1985 Apr;13(2):185–201. doi: 10.1007/BF01059398. [DOI] [PubMed] [Google Scholar]
- Shen D. D., Azarnoff D. L. Clinical pharmacokinetics of methotrexate. Clin Pharmacokinet. 1978 Jan-Feb;3(1):1–13. doi: 10.2165/00003088-197803010-00001. [DOI] [PubMed] [Google Scholar]
- Sinnett M. J., Groff G. D., Raddatz D. A., Franck W. A., Bertino J. S., Jr Methotrexate pharmacokinetics in patients with rheumatoid arthritis. J Rheumatol. 1989 Jun;16(6):745–748. [PubMed] [Google Scholar]
- Teresi M. E., Crom W. R., Choi K. E., Mirro J., Evans W. E. Methotrexate bioavailability after oral and intramuscular administration in children. J Pediatr. 1987 May;110(5):788–792. doi: 10.1016/s0022-3476(87)80025-2. [DOI] [PubMed] [Google Scholar]
- Tugwell P., Bennett K., Gent M. Methotrexate in rheumatoid arthritis. Indications, contraindications, efficacy, and safety. Ann Intern Med. 1987 Sep;107(3):358–366. doi: 10.7326/0003-4819-107-2-358. [DOI] [PubMed] [Google Scholar]
- Wan S. H., Huffman D. H., Azarnoff D. L., Stephens R., Hoogstraten B. Effect of route of administration and effusions on methotrexate pharmacokinetics. Cancer Res. 1974 Dec;34(12):3487–3491. [PubMed] [Google Scholar]
- Wang Y. M., Fujimoto T. Clinical pharmacokinetics of methotrexate in children. Clin Pharmacokinet. 1984 Jul-Aug;9(4):335–348. doi: 10.2165/00003088-198409040-00003. [DOI] [PubMed] [Google Scholar]
- Willkens R. F., Williams H. J., Ward J. R., Egger M. J., Reading J. C., Clements P. J., Cathcart E. S., Samuelson C. O., Jr, Solsky M. A., Kaplan S. B. Randomized, double-blind, placebo controlled trial of low-dose pulse methotrexate in psoriatic arthritis. Arthritis Rheum. 1984 Apr;27(4):376–381. doi: 10.1002/art.1780270403. [DOI] [PubMed] [Google Scholar]


