Abstract
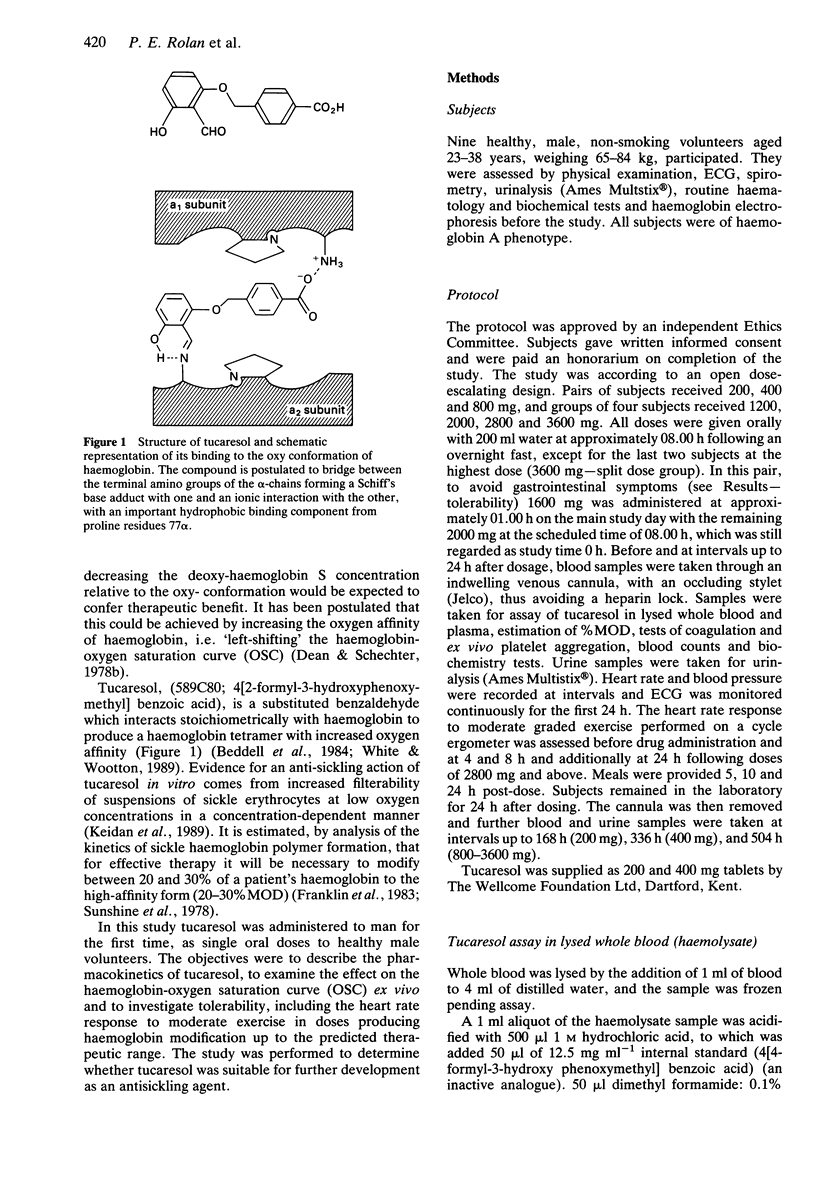
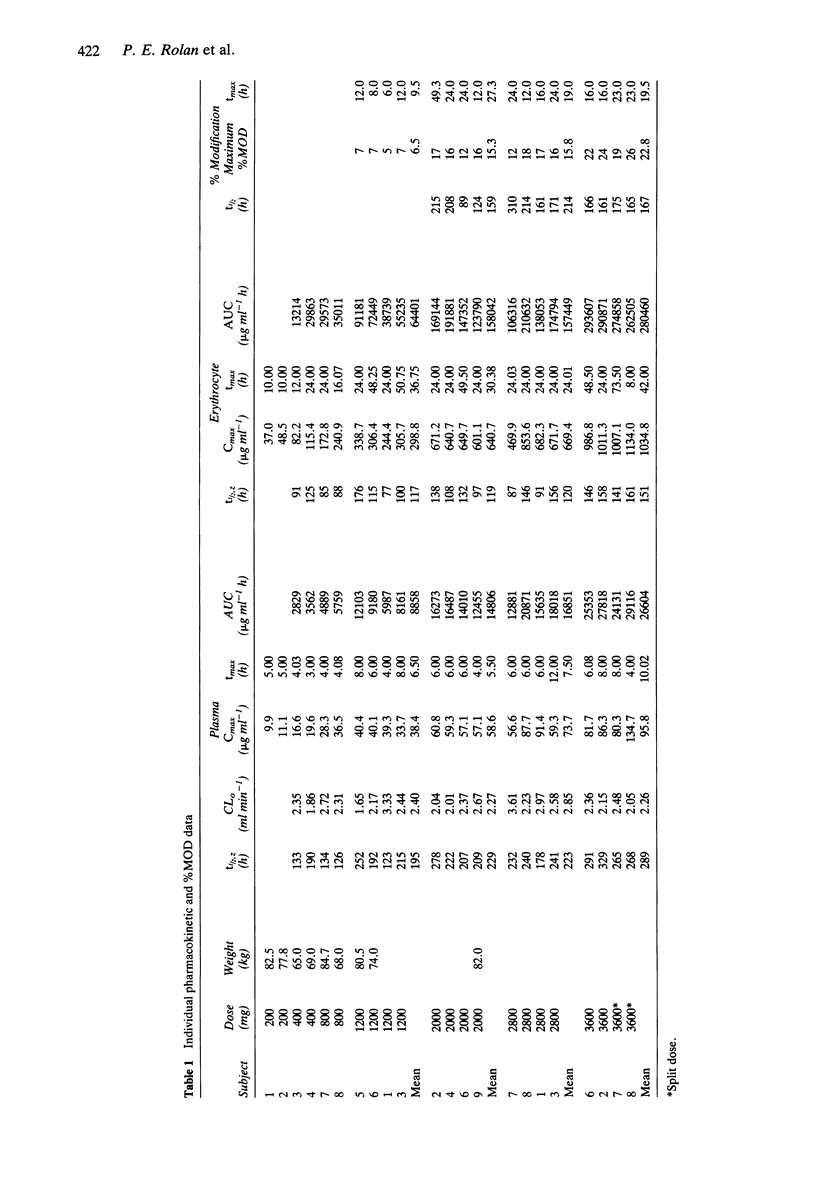
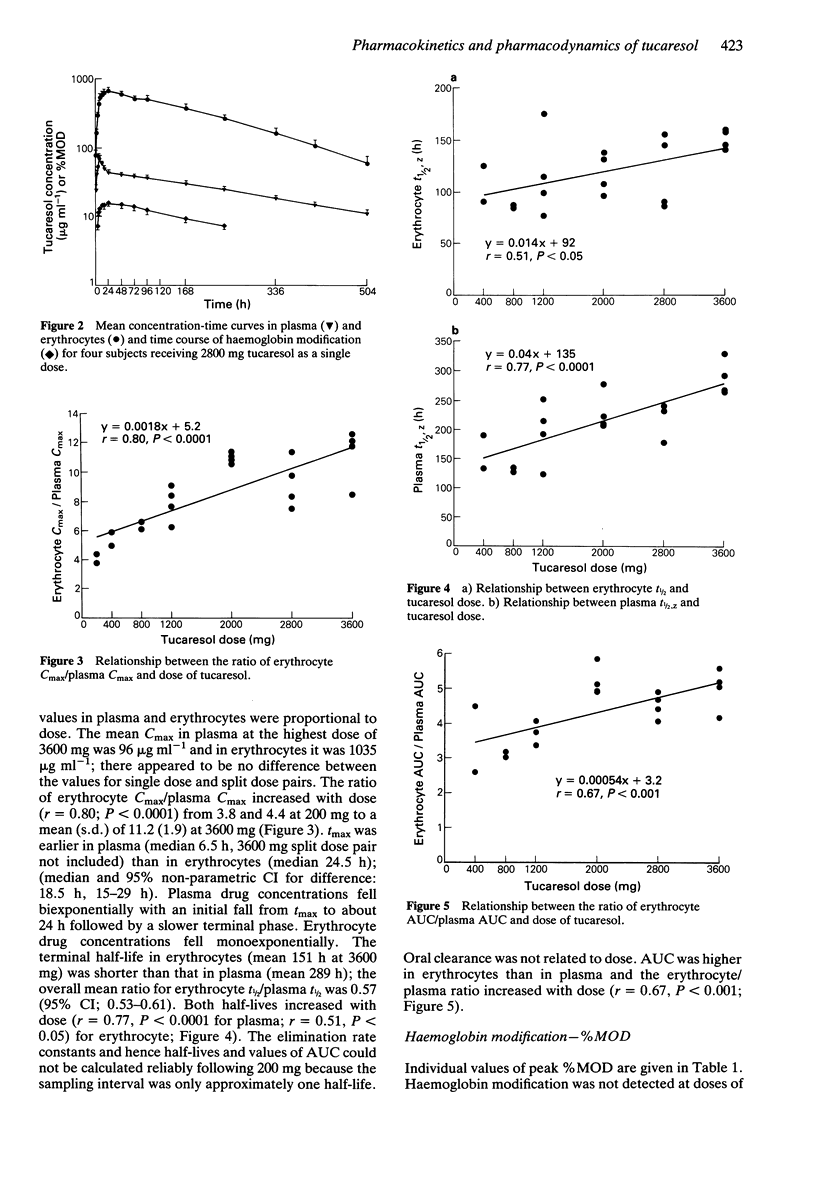
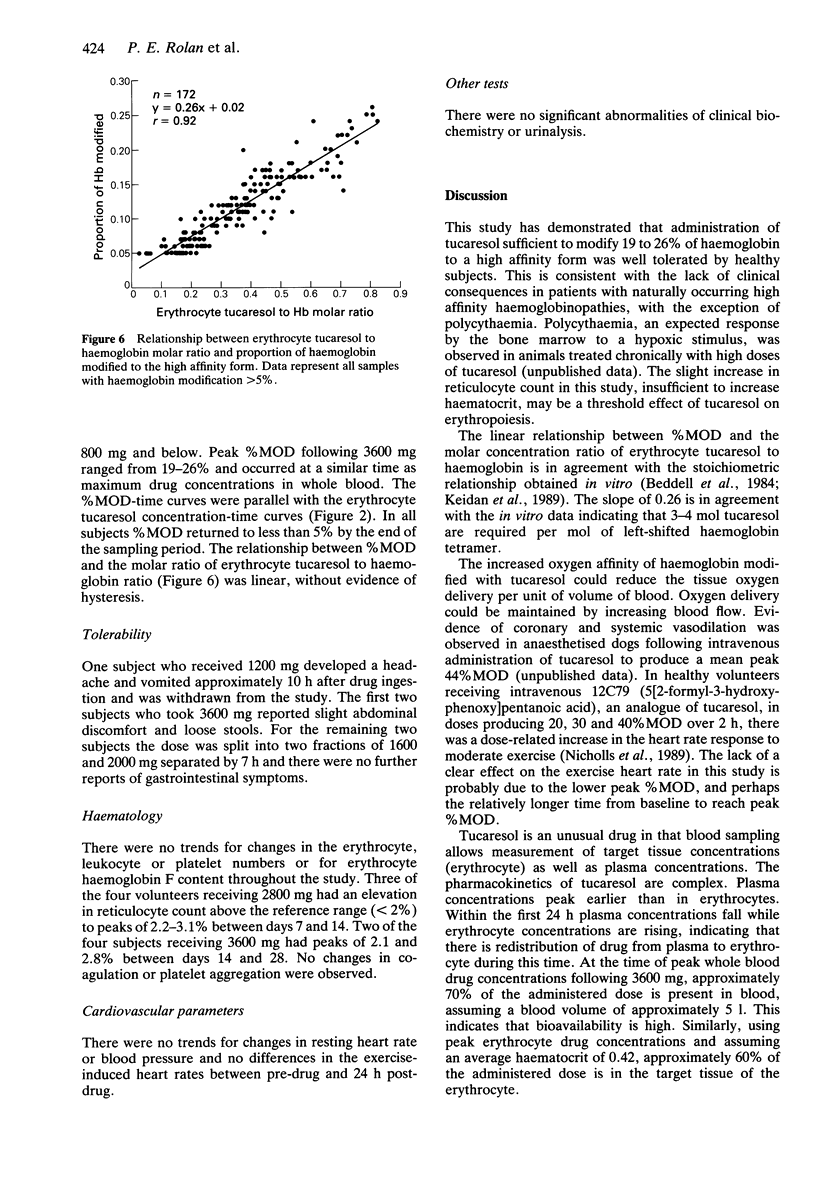
1. Tucaresol (589C80; 4[2-formyl-3-hydroxyphenoxymethyl] benzoic acid) interacts stoichiometrically with haemoglobin to increase oxygen affinity. By decreasing the proportion of insoluble deoxy sickle haemoglobin at capillary oxygen concentrations, tucaresol may be of therapeutic benefit in sickle cell anaemia. 2. In this study, which involved the first administration to man, the pharmacokinetics and pharmacodynamics of tucaresol were studied in healthy male volunteers following oral doses of 200-3600 mg. 3. Peak drug concentrations in plasma and erythrocytes were linearly related to dose; mean (s.d.) values were 95.8 (26.1) and 1035 (67) micrograms ml-1, respectively, at the highest dose. Median tmax in plasma was 6.5 h and in erythrocytes 24.5 h, when approximately 60% of the administered dose was in the target tissue. Plasma drug concentrations fell biexponentially with commencement of the apparent terminal elimination phase at approximately 24 h. The terminal elimination half-life from plasma increased with dose (r = 0.77; P < 0.0001) from 133-190 h at 400 mg to a mean (s.d.) of 289 (30) h at 3600 mg. Erythrocyte drug concentrations declined mono-exponentially with a half-life that was always shorter than the apparent terminal half-life in plasma: overall mean (95% CI) of t1/2 erythrocyte/t1/2 plasma ratio was 0.57 (0.53, 0.61). The erythrocyte AUC/plasma AUC ratio increased with dose (r = 0.67; P < 0.001). 4. The proportion of haemoglobin modified to a form with high oxygen affinity (%MOD) increased in a dose-related manner above doses of 800 mg reaching 19-26% after the 3600 mg dose.(ABSTRACT TRUNCATED AT 250 WORDS)
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baum K. F., Dunn D. T., Maude G. H., Serjeant G. R. The painful crisis of homozygous sickle cell disease. A study of the risk factors. Arch Intern Med. 1987 Jul;147(7):1231–1234. [PubMed] [Google Scholar]
- Beddell C. R., Goodford P. J., Kneen G., White R. D., Wilkinson S., Wootton R. Substituted benzaldehydes designed to increase the oxygen affinity of human haemoglobin and inhibit the sickling of sickle erythrocytes. Br J Pharmacol. 1984 Jun;82(2):397–407. doi: 10.1111/j.1476-5381.1984.tb10775.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Sickle-cell anemia: molecular and cellular bases of therapeutic approaches (first of three parts). N Engl J Med. 1978 Oct 5;299(14):752–763. doi: 10.1056/NEJM197810052991405. [DOI] [PubMed] [Google Scholar]
- Dean J., Schechter A. N. Sickle-cell anemia: molecular and cellular bases of therapeutic approaches (third of three parts). N Engl J Med. 1978 Oct 19;299(16):863–870. doi: 10.1056/NEJM197810192991605. [DOI] [PubMed] [Google Scholar]
- Franklin I. M., Rosemeyer M. A., Huehns E. R. Sickle cell disease: the proportion of liganded haemoglobin needed to prevent crises. Br J Haematol. 1983 Aug;54(4):579–587. doi: 10.1111/j.1365-2141.1983.tb02137.x. [DOI] [PubMed] [Google Scholar]
- Keidan A. J., Franklin I. M., White R. D., Joy M., Huehns E. R., Stuart J. Effect of BW12C on oxygen affinity of haemoglobin in sickle-cell disease. Lancet. 1986 Apr 12;1(8485):831–834. doi: 10.1016/s0140-6736(86)90941-4. [DOI] [PubMed] [Google Scholar]
- Keidan A. J., Sowter M. C., Johnson C. S., Marwah S. S., Stuart J. Pharmacological modification of oxygen affinity improves deformability of deoxygenated sickle erythrocytes: a possible therapeutic approach to sickle cell disease. Clin Sci (Lond) 1989 Apr;76(4):357–362. doi: 10.1042/cs0760357. [DOI] [PubMed] [Google Scholar]
- Lumley P., Humphrey P. P. A method for quantitating platelet aggregation and analyzing drug-receptor interactions on platelets in whole blood in vitro. J Pharmacol Methods. 1981 Sep;6(2):153–166. doi: 10.1016/0160-5402(81)90038-3. [DOI] [PubMed] [Google Scholar]
- Platt O. S. Is there treatment for sickle cell anemia? N Engl J Med. 1988 Dec 1;319(22):1479–1480. doi: 10.1056/NEJM198812013192210. [DOI] [PubMed] [Google Scholar]
- Sunshine H. R., Hofrichter J., Eaton W. A. Requirement for therapeutic inhibition of sickle haemoglobin gelation. Nature. 1978 Sep 21;275(5677):238–240. doi: 10.1038/275238a0. [DOI] [PubMed] [Google Scholar]


