Abstract
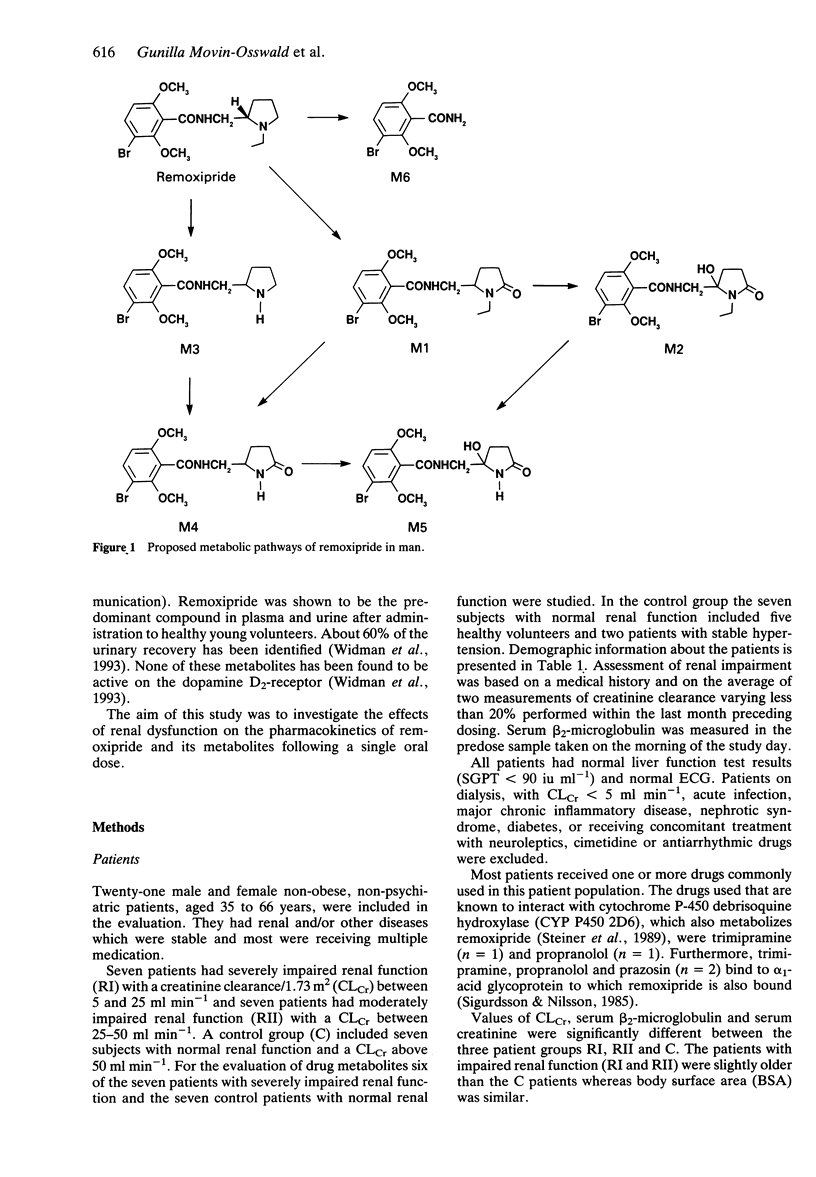
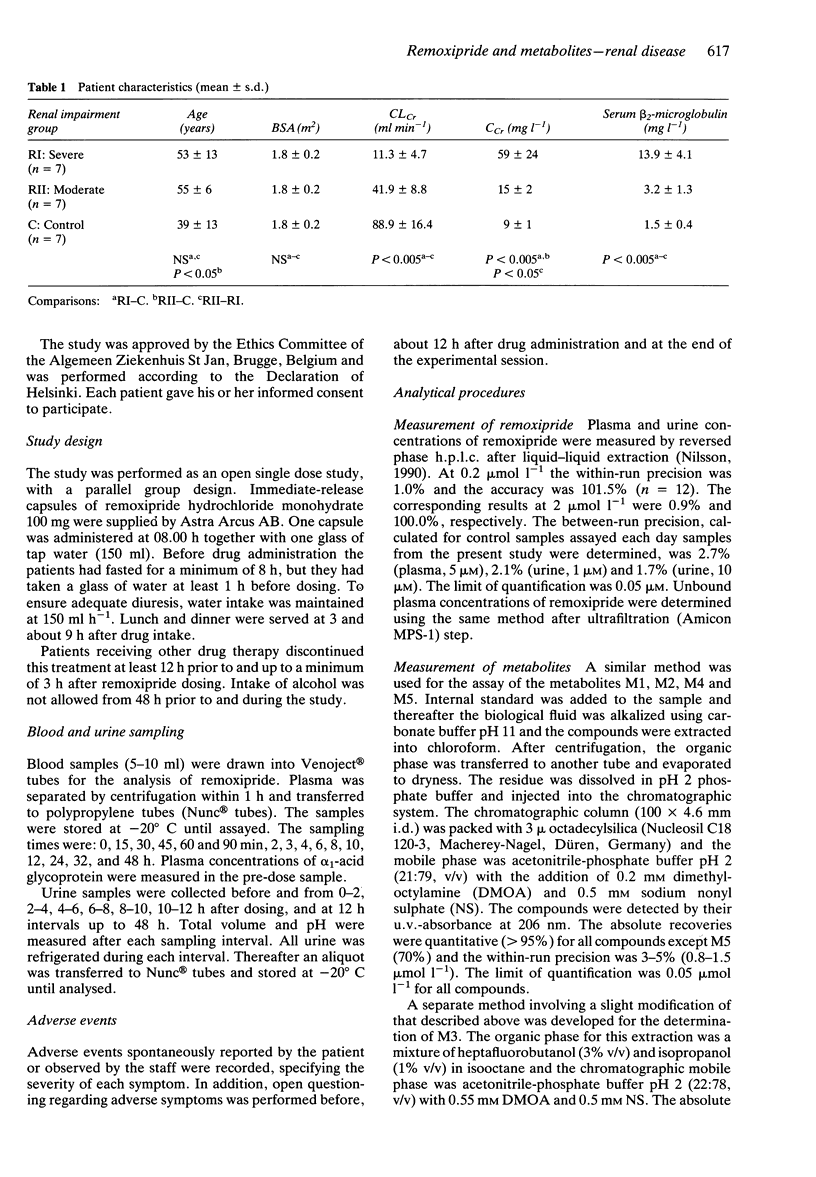
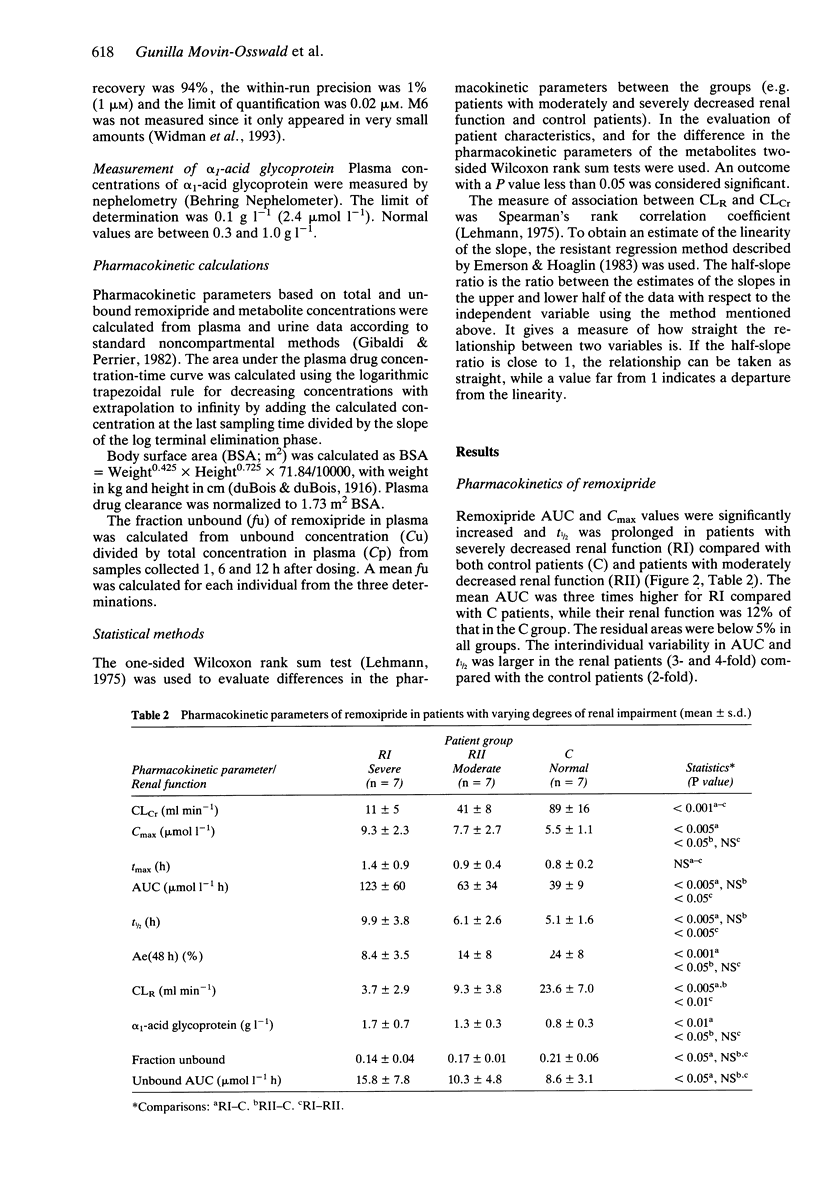
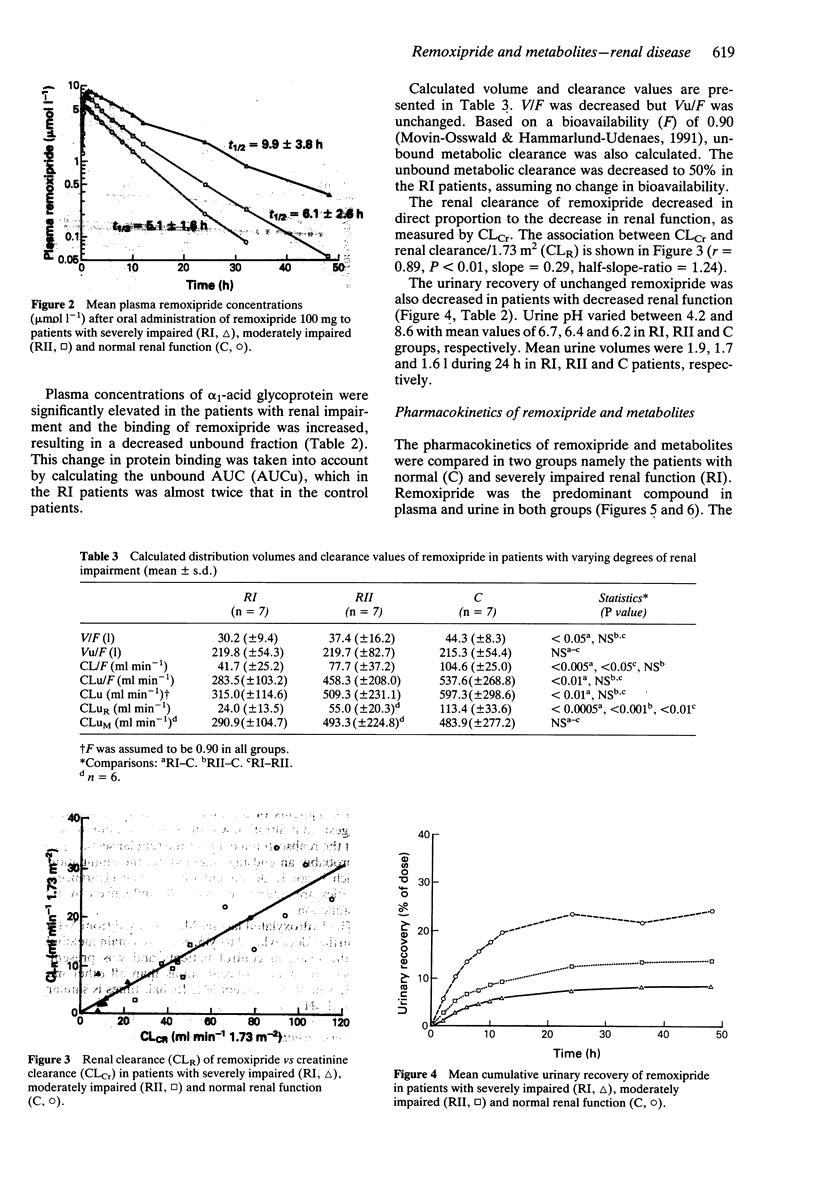
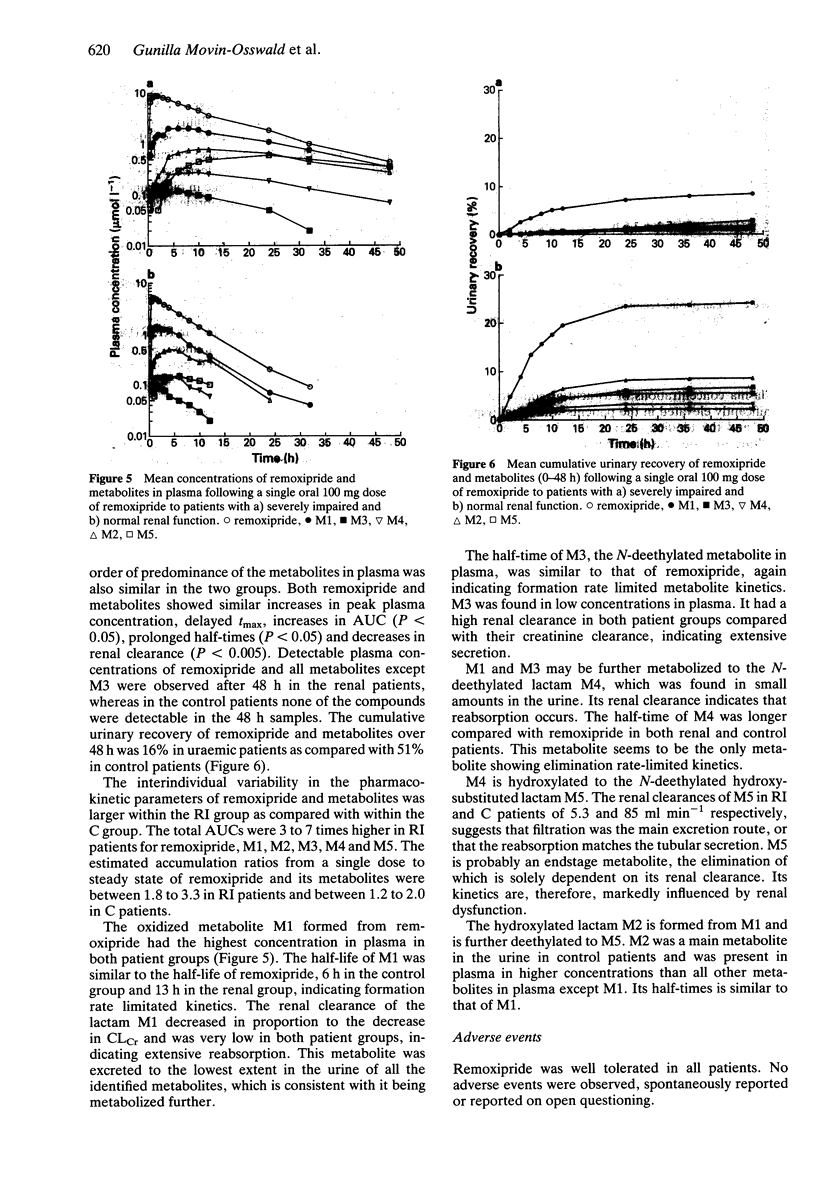
1. The pharmacokinetics of remoxipride, a new neuroleptic, were investigated in an open study with three parallel groups. Twenty-one patients with severely impaired (ClCr < 25 ml min-1), moderately impaired (ClCr 25-50 ml min-1) and normal (ClCr > 65 ml min-1) renal function were evaluated. A single oral dose of remoxipride hydrochloride 100 mg was administered, and blood and urine were collected over 48 h. Concentrations of remoxipride and metabolites were measured by h.p.l.c. 2. In patients with severely decreased renal function, the AUC and Cmax of remoxipride were increased significantly, and t1/2 was prolonged, as compared with the control patients. The renal clearance and urinary recovery of the unchanged drug were significantly diminished. 3. The unbound fraction of remoxipride in plasma was decreased in patients with renal failure, in association with a disease-related increase in alpha 1-acid glycoprotein. In spite of a 25% recovery of unchanged drug in the urine in patients with normal renal function, the AUC of unbound drug was twice as high in patients with severely impaired renal function. 4. A strong correlation between creatinine clearance and renal drug clearance was observed indicating a direct relationship between kidney function and the renal clearance of remoxipride. 5. Remoxipride was the predominant compound in plasma as well as in urine in patients with severely decreased as well as normal renal function. In patients with severely decrease renal function, remoxipride and all five pharmacologically inactive metabolites showed increased peak plasma concentrations, delayed tmax, increased AUC, prolonged half-lives and decreased renal clearance.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bianchetti G., Graziani G., Brancaccio D., Morganti A., Leonetti G., Manfrin M., Sega R., Gomeni R., Ponticelli C., Morselli P. L. Pharmacokinetics and effects of propranolol in terminal uraemic patients and in patients undergoing regular dialysis treatment. Clin Pharmacokinet. 1976;1(5):373–384. doi: 10.2165/00003088-197601050-00004. [DOI] [PubMed] [Google Scholar]
- Braun J., Sörgel F., Gluth W. P., Oie S. Does alpha 1-acid glycoprotein reduce the unbound metabolic clearance of disopyramide in patients with renal impairment? Eur J Clin Pharmacol. 1988;35(3):313–317. doi: 10.1007/BF00558271. [DOI] [PubMed] [Google Scholar]
- Grind M., Nilsson M. I., Nilsson L., Oxenstierna G., Sedvall G., Wahlén A. Remoxipride--a new potential antipsychotic compound. Tolerability and pharmacokinetics after single oral and intravenous administration in healthy male volunteers. Psychopharmacology (Berl) 1989;98(3):304–309. doi: 10.1007/BF00451679. [DOI] [PubMed] [Google Scholar]
- Lapierre Y. D., Nair N. P., Chouinard G., Awad A. G., Saxena B., Jones B., McClure D. J., Bakish D., Max P., Manchanda R. A controlled dose-ranging study of remoxipride and haloperidol in schizophrenia--a Canadian multicentre trial. Acta Psychiatr Scand Suppl. 1990;358:72–77. doi: 10.1111/j.1600-0447.1990.tb05293.x. [DOI] [PubMed] [Google Scholar]
- Lewander T., Westerbergh S. E., Morrison D. Clinical profile of remoxipride--a combined analysis of a comparative double-blind multicentre trial programme. Acta Psychiatr Scand Suppl. 1990;358:92–98. doi: 10.1111/j.1600-0447.1990.tb05297.x. [DOI] [PubMed] [Google Scholar]
- Movin-Osswald G., Hammarlund-Udenaes M. Remoxipride: pharmacokinetics and effect on plasma prolactin. Br J Clin Pharmacol. 1991 Sep;32(3):355–360. doi: 10.1111/j.1365-2125.1991.tb03911.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilsson L. B. Determination of remoxipride in plasma and urine by reversed-phase column liquid chromatography. J Chromatogr. 1990 Mar 16;526(1):139–150. doi: 10.1016/s0378-4347(00)82491-3. [DOI] [PubMed] [Google Scholar]
- Piafsky K. M., Borgá O., Odar-Cederlöf I., Johansson C., Sjöqvist F. Increased plasma protein binding of propranolol and chlorpromazine mediated by disease-induced elevations of plasma alpha1 acid glycoprotein. N Engl J Med. 1978 Dec 28;299(26):1435–1439. doi: 10.1056/NEJM197812282992604. [DOI] [PubMed] [Google Scholar]
- Sjöström P. A., Odlind B. G., Wolgast M. Extensive tubular secretion and reabsorption of creatinine in humans. Scand J Urol Nephrol. 1988;22(2):129–131. doi: 10.1080/00365599.1988.11690398. [DOI] [PubMed] [Google Scholar]
- Widerlöv E., Termander B., Nilsson M. I. Effect of urinary pH on the plasma and urinary kinetics of remoxipride in man. Eur J Clin Pharmacol. 1989;37(4):359–363. doi: 10.1007/BF00558500. [DOI] [PubMed] [Google Scholar]


