Abstract
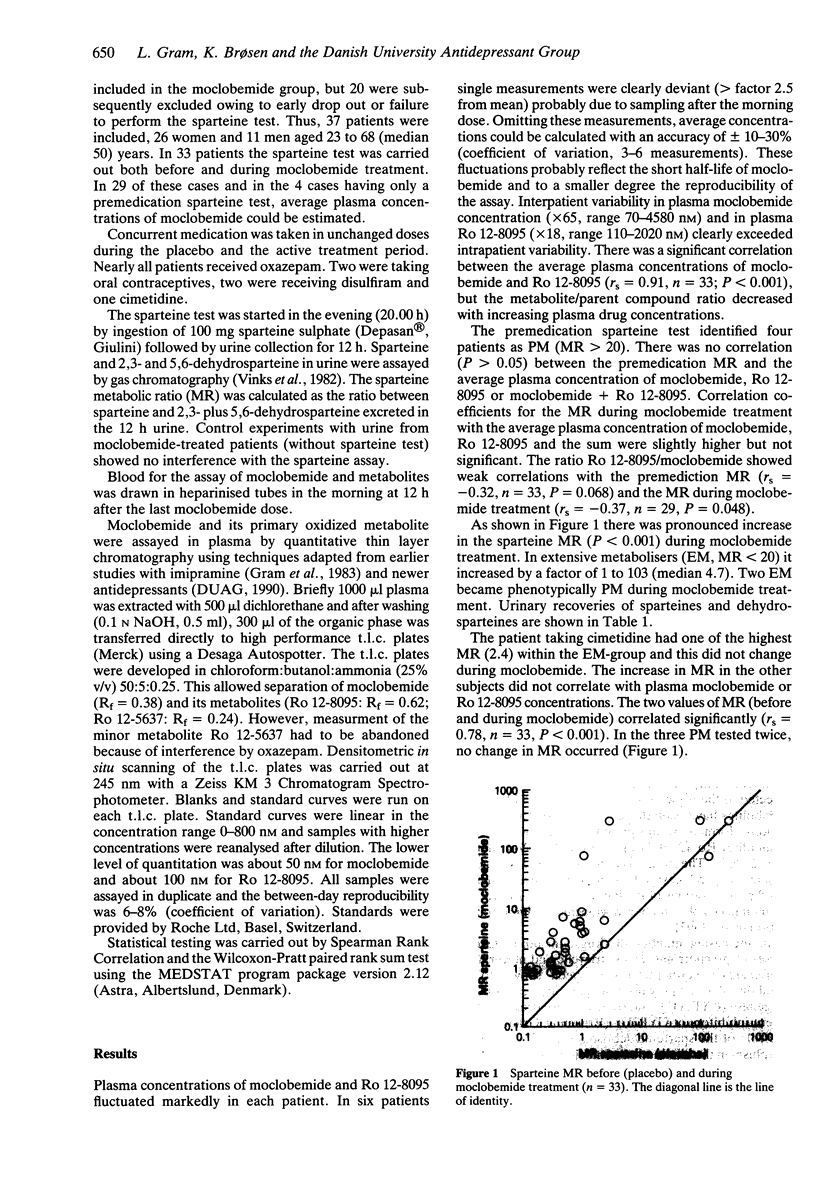
A sparteine test was carried out immediately before (n = 37) and during (n = 33) moclobemide treatment (200 mg twice daily) in 37 patients participating in a controlled clinical trial. The sparteine metabolic ratio (MR) did not correlate with the plasma concentration of moclobemide and/or its oxidized metabolite Ro 12-8095, and four sparteine poor metabolisers (PM, MR > 20) had plasma moclobemide concentrations similar to those in extensive metabolisers (EM, MR < 20). The Ro 12-8095/moclobemide ratio tended to correlate negatively with the sparteine MR before and during treatment (rs = -0.32, -0.37). During moclobemide treatment the sparteine MR rose substantially by a factor of 1-103 (median 4.7), and two EM became phenotypically PM. In the PM subjects as well as in one EM patient on cimetidine during both tests, no change in sparteine MR occurred.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brøsen K., Gram L. F. Clinical significance of the sparteine/debrisoquine oxidation polymorphism. Eur J Clin Pharmacol. 1989;36(6):537–547. doi: 10.1007/BF00637732. [DOI] [PubMed] [Google Scholar]
- Fitton A., Faulds D., Goa K. L. Moclobemide. A review of its pharmacological properties and therapeutic use in depressive illness. Drugs. 1992 Apr;43(4):561–596. doi: 10.2165/00003495-199243040-00009. [DOI] [PubMed] [Google Scholar]
- Gerber M. C., Tejwani G. A., Gerber N., Bianchine J. R. Drug interactions with cimetidine: an update. Pharmacol Ther. 1985;27(3):353–370. doi: 10.1016/0163-7258(85)90075-0. [DOI] [PubMed] [Google Scholar]
- Gram L. F., Bjerre M., Kragh-Sørensen P., Kvinesdal B., Molin J., Pedersen O. L., Reisby N. Imipramine metabolites in blood of patients during therapy and after overdose. Clin Pharmacol Ther. 1983 Mar;33(3):335–342. doi: 10.1038/clpt.1983.42. [DOI] [PubMed] [Google Scholar]
- Gram L. F., Debruyne D., Caillard V., Boulenger J. P., Lacotte J., Moulin M., Zarifian E. Substantial rise in sparteine metabolic ratio during haloperidol treatment. Br J Clin Pharmacol. 1989 Feb;27(2):272–275. doi: 10.1111/j.1365-2125.1989.tb05362.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guentert T. W., Tucker G., Korn A., Pfefen J. P., Haefelfinger P., Schoerlin M. P. Pharmacokinetics of moclobemide after single and multiple oral dosing with 150 milligrams 3 times daily for 15 days. Acta Psychiatr Scand Suppl. 1990;360:91–93. doi: 10.1111/j.1600-0447.1990.tb05345.x. [DOI] [PubMed] [Google Scholar]
- Inaba T., Jurima M., Mahon W. A., Kalow W. In vitro inhibition studies of two isozymes of human liver cytochrome P-450. Mephenytoin p-hydroxylase and sparteine monooxygenase. Drug Metab Dispos. 1985 Jul-Aug;13(4):443–448. [PubMed] [Google Scholar]
- Jauch R., Griesser E., Oesterhelt G., Arnold W., Meister W., Ziegler W. H., Guentert T. W. Biotransformation of moclobemide in humans. Acta Psychiatr Scand Suppl. 1990;360:87–90. doi: 10.1111/j.1600-0447.1990.tb05344.x. [DOI] [PubMed] [Google Scholar]
- Nielsen M. D., Brøsen K., Gram L. F. A dose-effect study of the in vivo inhibitory effect of quinidine on sparteine oxidation in man. Br J Clin Pharmacol. 1990 Mar;29(3):299–304. doi: 10.1111/j.1365-2125.1990.tb03639.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otton S. V., Inaba T., Kalow W. Competitive inhibition of sparteine oxidation in human liver by beta-adrenoceptor antagonists and other cardiovascular drugs. Life Sci. 1984 Jan 2;34(1):73–80. doi: 10.1016/0024-3205(84)90332-1. [DOI] [PubMed] [Google Scholar]
- Philip P. A., James C. A., Rogers H. J. The influence of cimetidine on debrisoquine 4-hydroxylation in extensive metabolizers. Eur J Clin Pharmacol. 1989;36(3):319–321. doi: 10.1007/BF00558167. [DOI] [PubMed] [Google Scholar]
- Raaflaub J., Haefelfinger P., Trautmann K. H. Single-dose pharmacokinetics of the MAO-inhibitor moclobemide in man. Arzneimittelforschung. 1984;34(1):80–82. [PubMed] [Google Scholar]
- Schoerlin M. P., Blouin R. A., Pfefen J. P., Guentert T. W. Comparison of the pharmacokinetics of moclobemide in poor and efficient metabolizers of debrisoquine. Acta Psychiatr Scand Suppl. 1990;360:98–100. doi: 10.1111/j.1600-0447.1990.tb05347.x. [DOI] [PubMed] [Google Scholar]
- Schoerlin M. P., Guentert T. W. Pharmakokinetik und Metabolismus reversibler MAO-A-Hemmer beim Menschen. Psychiatr Prax. 1989 Aug;16 (Suppl 1):11–17. [PubMed] [Google Scholar]
- Skjelbo E., Brøsen K. Inhibitors of imipramine metabolism by human liver microsomes. Br J Clin Pharmacol. 1992 Sep;34(3):256–261. doi: 10.1111/j.1365-2125.1992.tb04133.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinks A., Inaba T., Otton S. V., Kalow W. Sparteine metabolism in Canadian Caucasians. Clin Pharmacol Ther. 1982 Jan;31(1):23–29. doi: 10.1038/clpt.1982.4. [DOI] [PubMed] [Google Scholar]
- Wiesel F. A., Raaflaub J., Kettler R. Pharmacokinetics of oral moclobemide in healthy human subjects and effects on MAO-activity in platelets and excretion of urine monoamine metabolites. Eur J Clin Pharmacol. 1985;28(1):89–95. doi: 10.1007/BF00635714. [DOI] [PubMed] [Google Scholar]


