Abstract
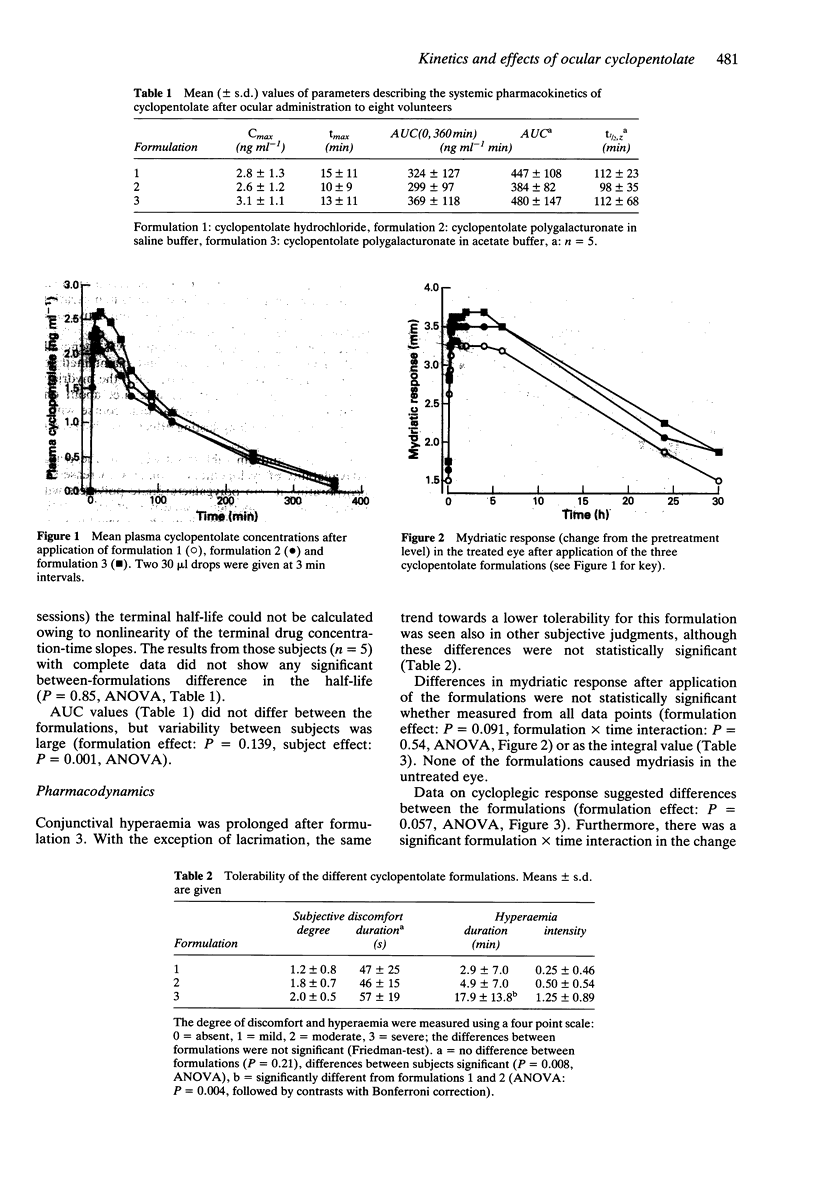
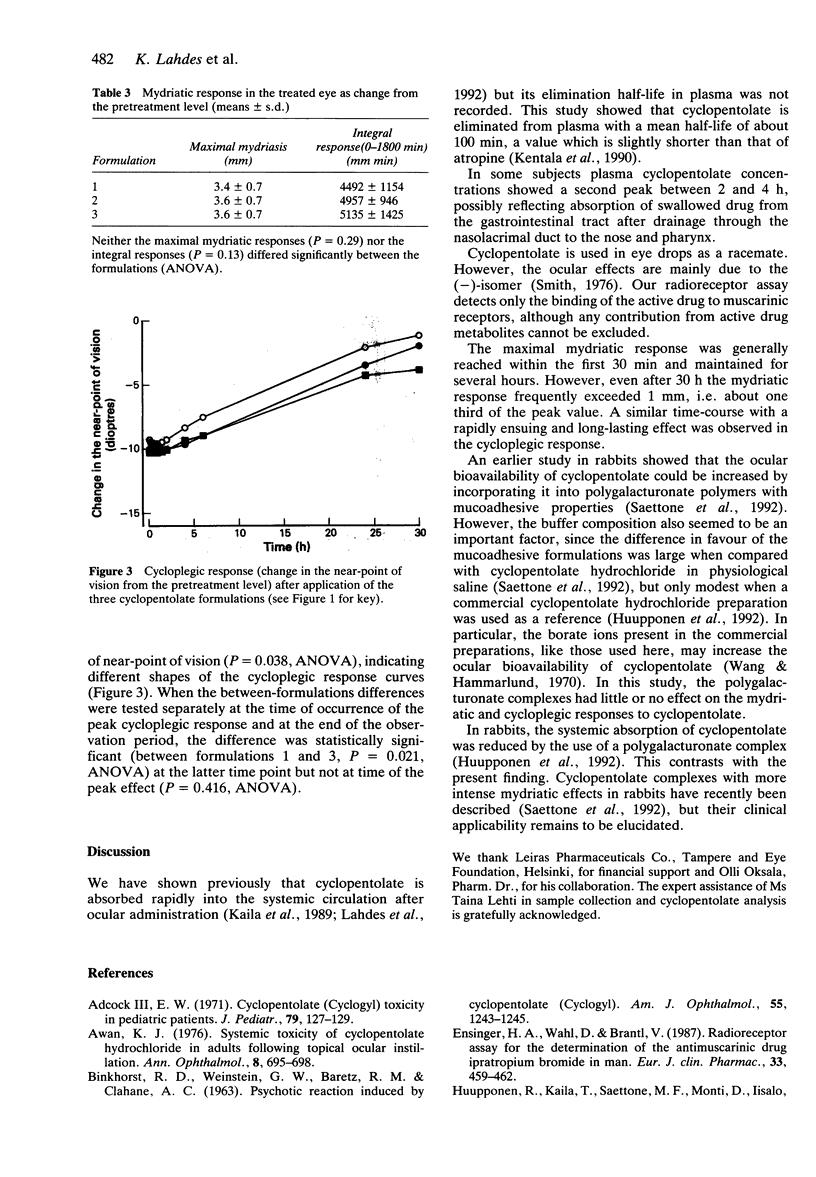
1. Eight volunteers received in randomized order two 30 microliters drops of either 1% w/v cyclopentolate hydrochloride or a corresponding amount of cyclopentolate polygalacturonate in saline or in acetate buffer in one eye. Cyclopentolate concentrations in plasma were measured by a radioreceptor assay. 2. Peak plasma drug concentrations of about 3 ng ml-1 occurred within 30 min after all formulations. Occasionally, a second concentration peak in plasma, probably reflecting drug absorption from the gastrointestinal tract, was seen after 2 h. The mean elimination half-life of cyclopentolate was 111 min when all subjects and formulations were considered together. There were no statistically significant differences between the formulations with respect to the time-course of plasma drug concentration. 3. The maximal mydriatic effect was reached within about 15 min and was maintained for several hours, often being 1/3 of its peak value after 30 h. Similarly, an intense cycloplegic response was achieved within a few minutes, the peak changes in the near-point of vision being 9 to 10 dioptres. The cycloplegic response was more intense after one of the polygalacturonate complexes, especially at later time points.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adcock E. W., 3rd Cyclopentolate (Cyclogyl) toxicity in pediatric patients. J Pediatr. 1971 Jul;79(1):127–129. doi: 10.1016/s0022-3476(71)80074-4. [DOI] [PubMed] [Google Scholar]
- Awan K. J. Adverse systemic reactions of topical cyclopentolate hydrochloride. Ann Ophthalmol. 1976 Jun;8(6):695–698. [PubMed] [Google Scholar]
- BINKHORST R. D., WEINSTEIN G. W., BARETZ R. M., CLAHANE A. C. Psychotic reaction induced by cyclopentolate (Cyclogyl). Results of pilot study and a double-blind study. Am J Ophthalmol. 1963 Jun;55:1243–1245. [PubMed] [Google Scholar]
- Ensinger H. A., Wahl D., Brantl V. Radioreceptor assay for determination of the antimuscarinic drug ipratropium bromide in man. Eur J Clin Pharmacol. 1987;33(5):459–462. doi: 10.1007/BF00544235. [DOI] [PubMed] [Google Scholar]
- Huupponen R., Kaila T., Saettone M. F., Monti D., Ilsalo E., Salminen L., Oksala O. The effect of some macromolecular ionic complexes on the pharmacokinetics and -dynamics of ocular cyclopentolate in rabbits. J Ocul Pharmacol. 1992 Spring;8(1):59–67. doi: 10.1089/jop.1992.8.59. [DOI] [PubMed] [Google Scholar]
- Kaila T., Huupponen R., Salminen L., Iisalo E. Systemic absorption of ophthalmic cyclopentolate. Am J Ophthalmol. 1989 May 15;107(5):562–564. doi: 10.1016/0002-9394(89)90515-1. [DOI] [PubMed] [Google Scholar]
- Kellner U., Esser J. Akute Psychose durch Intoxikation mit Cyclopentolat. Klin Monbl Augenheilkd. 1989 Jun;194(6):458–461. doi: 10.1055/s-2008-1046401. [DOI] [PubMed] [Google Scholar]
- Kentala E., Kaila T., Iisalo E., Kanto J. Intramuscular atropine in healthy volunteers: a pharmacokinetic and pharmacodynamic study. Int J Clin Pharmacol Ther Toxicol. 1990 Sep;28(9):399–404. [PubMed] [Google Scholar]
- Khurana A. K., Ahluwalia B. K., Rajan C., Vohra A. K. Acute psychosis associated with topical cyclopentolate hydrochloride. Am J Ophthalmol. 1988 Jan 15;105(1):91–91. doi: 10.1016/0002-9394(88)90128-6. [DOI] [PubMed] [Google Scholar]
- Lahdes K., Huupponen R., Kaila T., Ali-Melkkilä T., Salminen L., Saari M. Systemic absorption of ocular cyclopentolate in children. Ger J Ophthalmol. 1992;1(1):16–18. [PubMed] [Google Scholar]
- Salminen L. Review: systemic absorption of topically applied ocular drugs in humans. J Ocul Pharmacol. 1990 Fall;6(3):243–249. doi: 10.1089/jop.1990.6.243. [DOI] [PubMed] [Google Scholar]
- Sato E. H., de Freitas D., Foster C. S. Abuse of cyclopentolate hydrochloride (Cyclogyl) drops. N Engl J Med. 1992 May 14;326(20):1363–1364. [PubMed] [Google Scholar]
- Shihab Z. M. Psychotic reaction in an adult after topical cyclopentolate. Ophthalmologica. 1980;181(3-4):228–230. doi: 10.1159/000309057. [DOI] [PubMed] [Google Scholar]
- Smith S. A. Factors determining the potency of mydriatic drugs in man. Br J Clin Pharmacol. 1976 Jun;3(3):503–507. doi: 10.1111/j.1365-2125.1976.tb00628.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang E. S., Hammarlund E. R. Corneal absorption reinforcement of certain mydriatics. J Pharm Sci. 1970 Nov;59(11):1559–1563. doi: 10.1002/jps.2600591103. [DOI] [PubMed] [Google Scholar]
- Watsky M. A., Jablonski M. M., Edelhauser H. F. Comparison of conjunctival and corneal surface areas in rabbit and human. Curr Eye Res. 1988 May;7(5):483–486. doi: 10.3109/02713688809031801. [DOI] [PubMed] [Google Scholar]


