Abstract
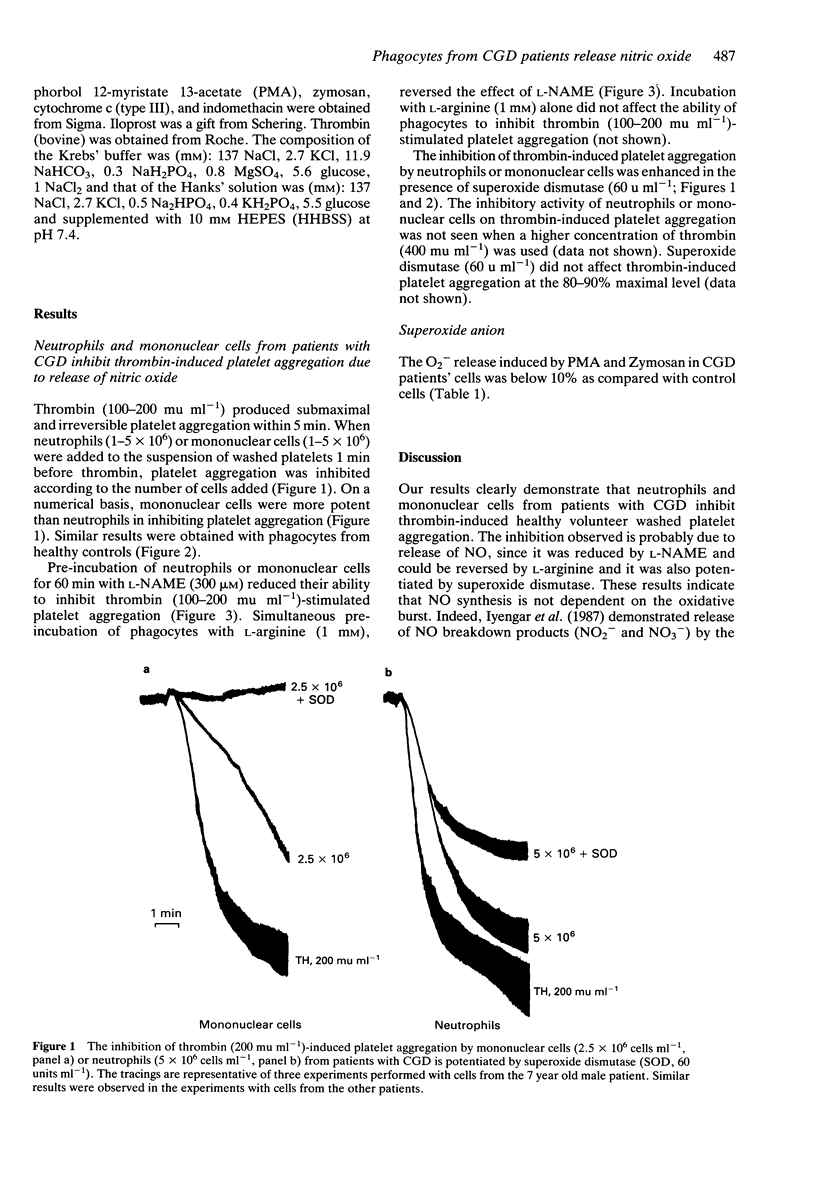
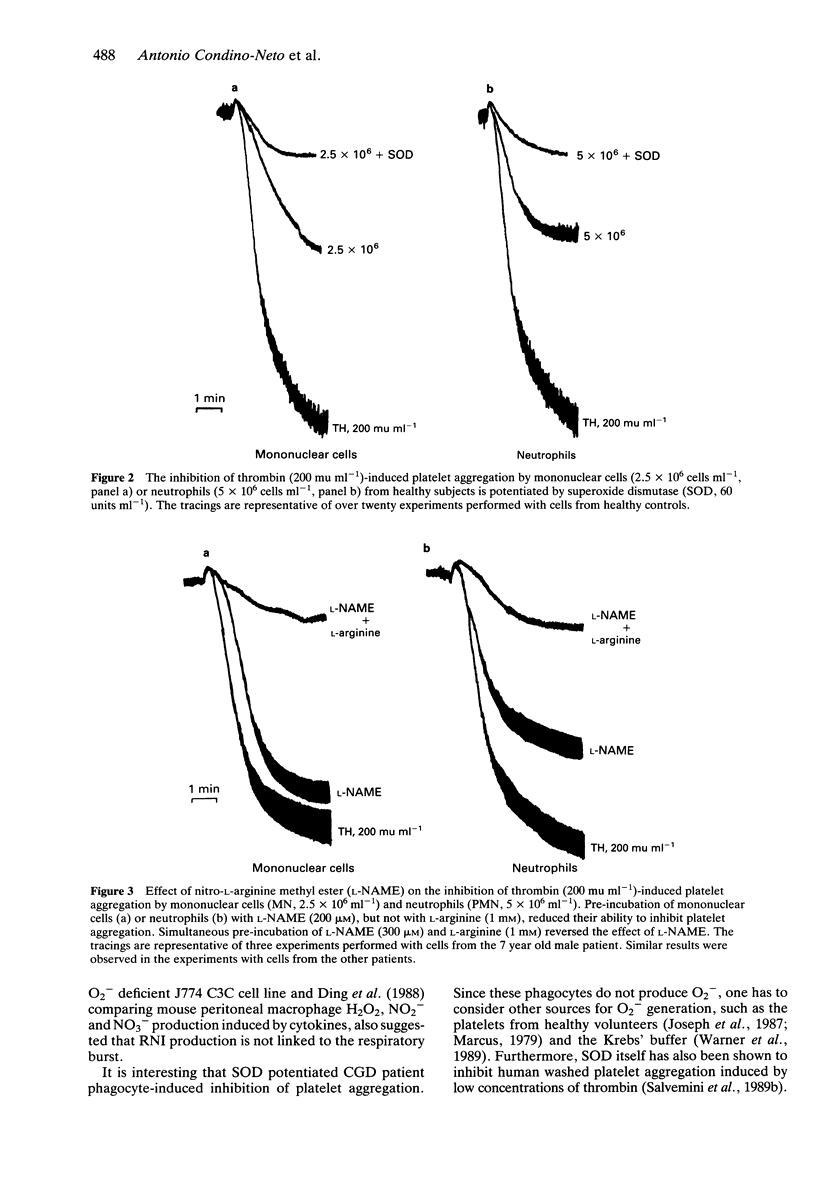
1. Chronic granulomatous disease (CGD) is a group of genetic disorders characterised by recurrent severe suppurative infections due to impaired microbial killing. The principal biochemical defect is an impairment in the production of reactive oxygen intermediates by phagocytes. 2. Nitric oxide (NO) is synthesised from the guanidino nitrogen atom(s) of L-arginine and has recently been proposed to be involved in defence mechanisms. The aim of this study was to investigate the involvement of the oxidative burst in the biosynthesis of NO by neutrophils and mononuclear cells from patients with CGD. 3. NO synthesis was assayed by the ability of neutrophils and mononuclear cells to inhibit thrombin-induced washed platelet aggregation while superoxide anion (O2-) production was measured spectrophotometrically by the superoxide dismutase inhibitable reduction of cytochrome c. 4. Neutrophils and mononuclear cells from patients with CGD released NO. This release was inhibited by nitro-L-arginine methyl ester but could be reversed by L-arginine. Zymosan- and PMA-induced O2- production was less than 10% as compared with healthy controls. 5. These results indicate that O2- production is not essential for NO synthesis in human leucocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BORN G. V., CROSS M. J. THE AGGREGATION OF BLOOD PLATELETS. J Physiol. 1963 Aug;168:178–195. doi: 10.1113/jphysiol.1963.sp007185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M., Curnutte J. T., McMurrich B. J. The particulate superoxide-forming system from human neutrophils. Properties of the system and further evidence supporting its participation in the respiratory burst. J Clin Invest. 1976 Oct;58(4):989–996. doi: 10.1172/JCI108553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (first of two parts). N Engl J Med. 1978 Mar 23;298(12):659–668. doi: 10.1056/NEJM197803232981205. [DOI] [PubMed] [Google Scholar]
- Babior B. M. The respiratory burst oxidase. Hematol Oncol Clin North Am. 1988 Jun;2(2):201–212. [PubMed] [Google Scholar]
- Bellinati-Pires R., Melki S. E., Colletto G. M., Carneiro-Sampaio M. M. Evaluation of a fluorochrome assay for assessing the bactericidal activity of neutrophils in human phagocyte dysfunctions. J Immunol Methods. 1989 May 12;119(2):189–196. doi: 10.1016/0022-1759(89)90395-5. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T., Babior B. M. Chronic granulomatous disease. Adv Hum Genet. 1987;16:229–297. doi: 10.1007/978-1-4757-0620-8_4. [DOI] [PubMed] [Google Scholar]
- Curnutte J. T. Classification of chronic granulomatous disease. Hematol Oncol Clin North Am. 1988 Jun;2(2):241–252. [PubMed] [Google Scholar]
- Curnutte J. T., Whitten D. M., Babior B. M. Defective superoxide production by granulocytes from patients with chronic granulomatous disease. N Engl J Med. 1974 Mar 14;290(11):593–597. doi: 10.1056/NEJM197403142901104. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Nathan C. F., Stuehr D. J. Release of reactive nitrogen intermediates and reactive oxygen intermediates from mouse peritoneal macrophages. Comparison of activating cytokines and evidence for independent production. J Immunol. 1988 Oct 1;141(7):2407–2412. [PubMed] [Google Scholar]
- English D., Andersen B. R. Single-step separation of red blood cells. Granulocytes and mononuclear leukocytes on discontinuous density gradients of Ficoll-Hypaque. J Immunol Methods. 1974 Aug;5(3):249–252. doi: 10.1016/0022-1759(74)90109-4. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Dinauer M. C., Jaffe H. S., Orkin S. H., Newburger P. E. Partial correction of the phagocyte defect in patients with X-linked chronic granulomatous disease by subcutaneous interferon gamma. N Engl J Med. 1988 Jul 21;319(3):146–151. doi: 10.1056/NEJM198807213190305. [DOI] [PubMed] [Google Scholar]
- Ezekowitz R. A., Orkin S. H., Newburger P. E. Recombinant interferon gamma augments phagocyte superoxide production and X-chronic granulomatous disease gene expression in X-linked variant chronic granulomatous disease. J Clin Invest. 1987 Oct;80(4):1009–1016. doi: 10.1172/JCI113153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gallin J. I., Buescher E. S., Seligmann B. E., Nath J., Gaither T., Katz P. NIH conference. Recent advances in chronic granulomatous disease. Ann Intern Med. 1983 Nov;99(5):657–674. doi: 10.7326/0003-4819-99-5-657. [DOI] [PubMed] [Google Scholar]
- Hegesh E., Shiloah J. Blood nitrates and infantile methemoglobinemia. Clin Chim Acta. 1982 Oct 27;125(2):107–115. doi: 10.1016/0009-8981(82)90187-5. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z. Macrophage cytotoxicity: role for L-arginine deiminase and imino nitrogen oxidation to nitrite. Science. 1987 Jan 23;235(4787):473–476. doi: 10.1126/science.2432665. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Taintor R. R., Vavrin Z., Rachlin E. M. Nitric oxide: a cytotoxic activated macrophage effector molecule. Biochem Biophys Res Commun. 1988 Nov 30;157(1):87–94. doi: 10.1016/s0006-291x(88)80015-9. [DOI] [PubMed] [Google Scholar]
- Hibbs J. B., Jr, Vavrin Z., Taintor R. R. L-arginine is required for expression of the activated macrophage effector mechanism causing selective metabolic inhibition in target cells. J Immunol. 1987 Jan 15;138(2):550–565. [PubMed] [Google Scholar]
- Holmes B., Page A. R., Good R. A. Studies of the metabolic activity of leukocytes from patients with a genetic abnormality of phagocytic function. J Clin Invest. 1967 Sep;46(9):1422–1432. doi: 10.1172/JCI105634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyengar R., Stuehr D. J., Marletta M. A. Macrophage synthesis of nitrite, nitrate, and N-nitrosamines: precursors and role of the respiratory burst. Proc Natl Acad Sci U S A. 1987 Sep;84(18):6369–6373. doi: 10.1073/pnas.84.18.6369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joseph M., Capron A., Tsicopoulos A., Ameisen J. C., Martinot J. B., Tonnel A. B. Platelet activation by IgE and aspirin. Agents Actions Suppl. 1987;21:169–177. doi: 10.1007/978-3-0348-7451-9_15. [DOI] [PubMed] [Google Scholar]
- Marcus A. J. Pathways of oxygen utilization by stimulated platelets and leukocytes. Semin Hematol. 1979 Jul;16(3):188–195. [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- McCall T. B., Feelisch M., Palmer R. M., Moncada S. Identification of N-iminoethyl-L-ornithine as an irreversible inhibitor of nitric oxide synthase in phagocytic cells. Br J Pharmacol. 1991 Jan;102(1):234–238. doi: 10.1111/j.1476-5381.1991.tb12159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCall T., Vallance P. Nitric oxide takes centre-stage with newly defined roles. Trends Pharmacol Sci. 1992 Jan;13(1):1–6. doi: 10.1016/0165-6147(92)90002-n. [DOI] [PubMed] [Google Scholar]
- McCord J. M., Fridovich I. Superoxide dismutase. An enzymic function for erythrocuprein (hemocuprein). J Biol Chem. 1969 Nov 25;244(22):6049–6055. [PubMed] [Google Scholar]
- Moore P. K., al-Swayeh O. A., Chong N. W., Evans R. A., Gibson A. L-NG-nitro arginine (L-NOARG), a novel, L-arginine-reversible inhibitor of endothelium-dependent vasodilatation in vitro. Br J Pharmacol. 1990 Feb;99(2):408–412. doi: 10.1111/j.1476-5381.1990.tb14717.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer R. M., Ashton D. S., Moncada S. Vascular endothelial cells synthesize nitric oxide from L-arginine. Nature. 1988 Jun 16;333(6174):664–666. doi: 10.1038/333664a0. [DOI] [PubMed] [Google Scholar]
- ROSSI F., ZATTI M. CHANGES IN THE METABOLIC PATTERN OF POLYMORPHO-NUCLEAR LEUCOCYTES DURING PHAGOCYTOSIS. Br J Exp Pathol. 1964 Oct;45:548–559. [PMC free article] [PubMed] [Google Scholar]
- Radomski M., Moncada S. An improved method for washing of human platelets with prostacyclin. Thromb Res. 1983 May 15;30(4):383–389. doi: 10.1016/0049-3848(83)90230-x. [DOI] [PubMed] [Google Scholar]
- Rimele T. J., Sturm R. J., Adams L. M., Henry D. E., Heaslip R. J., Weichman B. M., Grimes D. Interaction of neutrophils with vascular smooth muscle: identification of a neutrophil-derived relaxing factor. J Pharmacol Exp Ther. 1988 Apr;245(1):102–111. [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Gryglewski R. J., Vane J. R. Human neutrophils and mononuclear cells inhibit platelet aggregation by releasing a nitric oxide-like factor. Proc Natl Acad Sci U S A. 1989 Aug;86(16):6328–6332. doi: 10.1073/pnas.86.16.6328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salvemini D., de Nucci G., Sneddon J. M., Vane J. R. Superoxide anions enhance platelet adhesion and aggregation. Br J Pharmacol. 1989 Aug;97(4):1145–1150. doi: 10.1111/j.1476-5381.1989.tb12572.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Selvaraj R. J., Sbarra A. J. Relationship of glycolytic and oxidative metabolism to particle entry and destruction in phagocytosing cells. Nature. 1966 Sep 17;211(5055):1272–1276. doi: 10.1038/2111272a0. [DOI] [PubMed] [Google Scholar]
- Stuehr D. J., Gross S. S., Sakuma I., Levi R., Nathan C. F. Activated murine macrophages secrete a metabolite of arginine with the bioactivity of endothelium-derived relaxing factor and the chemical reactivity of nitric oxide. J Exp Med. 1989 Mar 1;169(3):1011–1020. doi: 10.1084/jem.169.3.1011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Induction of nitrite/nitrate synthesis in murine macrophages by BCG infection, lymphokines, or interferon-gamma. J Immunol. 1987 Jul 15;139(2):518–525. [PubMed] [Google Scholar]
- Stuehr D. J., Marletta M. A. Mammalian nitrate biosynthesis: mouse macrophages produce nitrite and nitrate in response to Escherichia coli lipopolysaccharide. Proc Natl Acad Sci U S A. 1985 Nov;82(22):7738–7742. doi: 10.1073/pnas.82.22.7738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tauber A. I., Borregaard N., Simons E., Wright J. Chronic granulomatous disease: a syndrome of phagocyte oxidase deficiencies. Medicine (Baltimore) 1983 Sep;62(5):286–309. [PubMed] [Google Scholar]
- Warner T. D., de Nucci G., Vane J. R. Comparison of the survival of endothelium-derived relaxing factor and nitric oxide within the isolated perfused mesenteric arterial bed of the rat. Br J Pharmacol. 1989 Jul;97(3):777–782. doi: 10.1111/j.1476-5381.1989.tb12016.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


