Abstract
Objective
Constrictive remodeling accounts for lumen loss in postangioplasty restenosis. Matrix metalloproteinase-9 (MMP-9) has been shown to prevent constrictive remodeling in vivo. To investigate potential mechanisms for this observation, we investigated the role of MMP-9 in smooth muscle cell (SMC)-mediated collagen gel contraction, an in vitro model of constrictive remodeling.
Methods
Fischer rat SMCs were stably transfected with a construct-expressing rat-MMP-9 under the control of a tetracycline (Tet)-off promoter. SMCs were seeded in type I collagen gels (2.4 mg/ml) in the presence or not of tetracycline (1 μg/ml), and gel contraction was defined as the percentage of retraction of the collagen gel. The depletion of MMP-9 was obtained by using siRNA targeting MMP-9 mRNA or a blocking antibody.
Results
Gel contraction was significantly reduced at all times when MMP-9 was overexpressed (Tet−) as compared with the control condition (Tet+). However, MMP-9 depletion of control (Tet+) SMCs (using siRNA or antibody) also inhibited gel contraction. To resolve the apparent discrepancy and determine if MMP-9 exerts a dose-dependent biphasic effect on gel contraction, conditioned medium and purified rat-MMP-9 were prepared. Gel contraction was significantly increased by addition of 0.8 ng/ml of MMP-9, while high concentrations of MMP-9 (≥100 ng/ml) inhibited contraction. The addition of BB94 and TIMP-1 did not alter the inhibitory or stimulatory effect of MMP-9.
Conclusions
Our data suggest that MMP-9, independent of its proteolytic function, has a biphasic effect on SMC-mediated collagen gel contraction. Understanding the different roles of MMP-9 should allow the development of better therapeutic strategies for restenotic vascular disease.
Keywords: Matrix metalloproteinase-9, Contraction, Rat, Smooth muscle cell, Remodeling
1. Introduction
Arterial remodeling is an important aspect of atherosclerosis and restenosis after interventions such as angioplasty. It has become the object of intense research since vascular remodeling is one of the key processes regulating lumen diameter after vascular injury and since restenosis is a major cause of morbidity in humans [1]. Expansive (outward or positive) remodeling helps to maintain luminal cross-sectional area during early phases of atherosclerotic plaque growth, but constrictive (inward or negative) remodeling in late phases of plaque growth and after angioplasty contributes to luminal narrowing. Multiple events may participate in remodeling, including smooth muscle cell (SMC) proliferation and migration, apoptosis, and changes in cell/matrix interactions [2–5]. Extracellular matrix degrading proteinases [e.g., matrix metalloproteinases (MMPs)] modify matrix composition and properties, both facets being essential for cell/matrix interactions. MMPs have been shown to be involved in arterial remodeling using animal models [6,7]. Others and we previously demonstrated that MMP-9 prevents constrictive remodeling and is necessary for outward remodeling using different in vivo models [8,9]. However, MMP-9 was also reported to be needed for constrictive remodeling in a MMP-9 genetic deficiency mouse carotid tie-off model [10]. To understand this apparent discrepancy, we investigated the effect of MMP-9 on SMC-mediated collagen gel contraction, an in vitro model of constrictive remodeling. The use of this model is supported by the significant contribution of tissue contraction in lumen constriction [11,12]. We have found that MMP-9 has a biphasic effect on SMC-mediated collagen gel contraction. At low concentrations, MMP-9 stimulates contraction, while at high concentrations, MMP-9 inhibits contraction. Of particular interest, proteolytic activity of MMP-9 is not required for these effects.
2. Methods
2.1. Materials
siRNA for rat MMP-9 was purchased from Eurogentec (Seraing, Belgium). Collagen (Vitrogen) was purchased from Collagen (Fremont, CA). An antibody against human MMP-9, and cross reacting with rat-MMP-9, was purchased from The Binding Site (Birmingham, UK), and other chemical reagents were purchased from Sigma (St. Louis, MO).
2.2. Cell Culture
Fischer 344 rat aortic smooth muscle cells [SMC(tTA-MMP-9)] stably transfected with tTA protein and rat MMP-9, which are both under control of the Tet operator, were prepared, as described previously [8], and in accordance with state and federal laws and under protocols approved by the University of Washington Animal Care and Use Committee. Animal care complied with the Guide for the Care and Use of Laboratory Animals issued by the Institute of Laboratory Animal Resources (NIH publication 85–23, revised 1996). The transfected cells express low levels of MMP-9 in the presence of tetracycline and high levels of MMP-9 in the absence of tetracycline. The cells were maintained in DMEM with 10% calf serum and 1 μg/ml tetracycline. To prepare conditioned medium, cells were washed twice with PBS and maintained in 10% serum with or without tetracycline for 24 h. Cells were then washed three times with PBS and maintained in medium without serum and with or without tetracycline. After 48 h, conditioned medium was collected.
2.3. Assay for collagen gel contraction by SMCs
SMCs(tTA-MMP-9) were maintained in 10% serum with or without tetracycline for 24 h prior to seeding in type I collagen at a final concentration of 2.5−105 cells/ml and 2.5 mg collagen/ml. The mixture of cells and collagen was poured into 24-well tissue culture plates (1 ml per well) that were precoated with 1% agarose and allowed to polymerize at 37 °C in a 5% CO2 incubator for 1 h. Then, 1 ml of DMEM with 10% serum with or without tetracycline was added. Gel contraction was measured every 24 h by scanning the plates with a flat bed scanner and determining area of the gel using NIH ImageQuant. The area of an empty well (2 cm2) was measured as a reference.
2.4. SMC proliferation, DNA quantification, and trypan blue exclusion test
After 2 and 5 days of gel contraction, 1 μCi/ml of [3H] thymidine was added to the medium for 24 h. As a control, parallel SMC-gel cultures were subjected to three cycles of freezing at −80 °C and thawing at 37 °C before radiolabeling. After radiolabeling, medium was removed and 1 ml of 20% TCA was added overnight at 4 °C. Gels were then vacuum-collected on 0.45 μm filters (Millipore) and washed several times with cold 10% TCA. Filters were then incubated overnight in 1 M NaOH at 37 °C. Solubilized radioactivity was measured by liquid scintillation counting. DNA quantification within the SMC-populated collagen gel at day 6 was performed using DNA assay, as described by Labarca and Paigen [13]. The ratio between living and dead cells was also determined using trypan blue exclusion test.
2.5. MMP-9 activity assay
To investigate the effect of metalloproteinase inhibitors (BB-94 and TIMP-1) as well as MMP-9 antibody on rat-MMP-9 activity, in vitro activity assays were performed, as described by Oltenfreiter et al. [14]. Briefly, the initial rate of cleavage of a fluorescent substrate Mca–Pro–Leu–Gly–Leu–Dpa–Ala–Arg–NH2 (4.5 μM) by purified rat-MMP-9 (150 nM) previously activated by using APMA ( p-aminophenylmercuric acetate, 1 mM) was measured over 20 min in the absence or presence of MMP inhibitors (BB-94, 0–10 nM; TIMP-1, ratio TIMP-1/MMP-9 of 2 and 20) or MMP-9 antibody (0–100 μg/ml). Results are expressed in percentage of inhibition.
2.6. siRNA transfection
To inhibit MMP-9 synthesis, two 21-nucleotide RNAs were chemically synthesized and purified by reverse-phase HPLC (Eurogentec). The sequences for MMP-9 were 5′-CAUCACCUAUUGGAUCCAAdTdT-3′ and 5′-UUGGAUCCAAUAGGUGAUGdTdT-3′, and for a scrambled control, they were 5′-AUACUUACGCACGCUCCAATT-3′ and 5′-UUGGAGCGUGCGUAAGUAUTT-3′. Each pair of oligonucleotides was annealed at a concentration of 20 μM in 10 mM Tris–HCl (pH 7.5), 50 mM NaCl, 1 mM EDTA. SMCs at 50% confluence in 10-cm dishes were transfected with 5 nM siRNA, using the calcium phosphate-precipitation procedure for 15 h [15]. Cells were used in the collagen gel contraction assay 24 h after transfection.
2.7. Rat MMP-9 purification
Rat MMP-9 purification was performed using gelatin–Sepharose chromatography followed by Con A–Sepharose affinity chromatography according to Morodomi et al. [16]. Briefly, serum-free conditioned medium of rat SMC overexpressing MMP-9 was applied to a column of gelatin–Sepharose equilibrated with TNC buffer [50 mM Tris–HCl (pH 7.5), 0.15 M NaCl, 10 mM CaCl2, 0.02% NaN3, 0.05% Brij 35]. After extensive washing, elution was performed using 5% (v/v) DMSO in TNC buffer. The collected fractions were then applied to a Con A–Sepharose column equilibrated with TNC buffer. After washing with TNC buffer, MMP-9 was eluted in 1 M α-methyl-d-mannoside in TNC buffer. The purified samples were dialyzed against DMEM and analysed by gelatin zymography and ELISA to confirm the depletion of MMP-2 and fibronectin, respectively, two other gelatin-binding proteins.
2.8. MMP-9 depletion of fetal calf serum by affinity chromatography
Gelatin–Sepharose chromatography in TNC buffer [50 mM Tris–HCl (pH 7.5), 0.15 M NaCl, 10 mM CaCl2, 0.02% NaN3, 0.05% Brij 35] was used to remove MMP-9 from fetal calf serum according to Morodomi et al. [16]. The unbound fraction (MMP-2/MMP-9 depleted serum) and the 5% DMSO elution fraction (mainly MMP-2 and MMP-9) were dialyzed against DMEM.
2.9. Zymography
Equal amounts of conditioned medium from identical numbers of cells grown under serum-free conditions for 48 h were loaded onto gelatin SDS polyacrylamide gels, and zymography was performed as described [17].
2.10. Statistics
Results are given as mean ± S.E.M. of n independent experiments. Student’s t test or one-way ANOVA were performed for comparisons between groups as indicated. P ≤ 0.05 was considered as significant.
3. Results
3.1. Overexpression of MMP-9 inhibits collagen gel contraction by SMCs
In confirmation of a previous work [8], we observed that conditioned medium collected from rat SMC(tTA-MMP-9) cells cultured in the absence of tetracycline (Tet−) showed a strong expression of proMMP-9 (~103 kDa) compared to the expression of MMP-9 in the presence of tetracycline (Tet+; Fig. 1). ProMMP-2 (doublet at ~67 kDa) was present and not altered by tetracycline. Overnight incubation of gelatin zymograms in the activation buffer containing tetracycline at doses up to 50 μg/ml did not demonstrate any significant effect of tetracycline on MMP activity (data not shown).
Fig. 1.

Gelatin zymography of serum-free medium conditioned 48 h by cells transfected with tetracycline-driven rat MMP-9 in the presence (Tet+) or absence (Tet−) of tetracycline and concentrated 20-fold. Gelatinolytic activity is indicated by clear zones against a dark background of stained substrate.
To investigate the effect of the overexpression of MMP-9 on SMC contractile activity, we performed a collagen gel contraction assay in the presence or absence of tetracycline. In control conditions (Tet+), collagen gel contraction started after 2 days, while when MMP-9 was overexpressed (Tet−), contraction began after 3 days (Fig. 2). Gel contraction was significantly reduced at all times when MMP-9 was overexpressed.
Fig. 2.

Overexpression of MMP-9 inhibits collagen gel contraction by rat SMC. Effect of overexpression of MMP-9 (Tet−) on collagen gel contraction by rat-SMC(tTA-MMP-9). The illustration of the collagen gel contractions in Tet+ and Tet− condition is representative of a day 6 situation. Data are expressed as percent of gel contraction. *P < 0.05 (n=4).
A possible role for calf serum MMP-9 in SMC-mediated collagen gel contraction was then examined. No significant difference was found in gel contraction when 10% normal serum, 10% MMP-9-depleted serum, or 10% reconstituted serum (depleted serum repleted with the MMP-9 containing bound fraction) was used (data not shown).
To investigate a possible effect of MMP-9 on cell proliferation, we measured [3H] thymidine incorporation into DNA by SMC either overexpressing MMP-9 (Tet−) or not (Tet+). There was no significant difference in the DNA synthesis by SMCs in collagen gels between the two experimental conditions at days 2 and 5 (day 2: 128,955 ± 4953 and 129,027 ± 39,109 CPM, Tet− and Tet+, respectively; day 5: 170,526 ± 2162 and 181,510 ± 31,869 CPM, Tet− and Tet+, respectively, n=3).
The effect of MMP-9 on cell death was also investigated by measuring total DNA and the living cells/dead cells ratio after staining cells with trypan blue. There was no significant difference in the amount of DNA in SMC-populated collagen gels between the Tet− and Tet+ conditions at day 6 (483 ± 60 and 503 ± 75 ng of DNA, Tet− and Tet+, respectively, n=3). The living cells/dead cells ratio in SMC-populated collagen gels did not vary significantly between the two experimental conditions at day 6 (6 ± 2 and 7 ± 3, Tet− and Tet+, respectively, n=3).
The specificity of the inhibitory effect of MMP-9 on gel contraction was investigated by using an MMP-9 antibody and by knocking-down the MMP-9 mRNA with siRNA. The addition of anti-MMP-9 (50–200 μg/ml) to SMCs overexpressing MMP-9 (Tet−) restored normal contraction of the collagen gels with an optimal effect obtained with 100 μg/ml of antibody (Fig. 3A). SMCs overexpressing MMP-9 (Tet−) were transfected with siRNA targeting MMP-9 mRNA. The level of MMP-9 was reduced to about 15% of the control level (siRNA scramble) up to 3-day post transfection and to about 20% after 5 days of transfection (Fig. 2B). The treatment by siRNA against MMP-9 restored collagen gel contraction to a level similar to that observed with cells that do not overexpress MMP-9 (Tet+; Fig. 3C). Thus, blockade of MMP-9 by two independent procedures reversed the inhibitory effect of MMP-9 on collagen gel contraction.
Fig. 3.

Reversal of MMP-9-mediated inhibition of collagen gel contraction by an MMP-9 antibody or siRNA targeting MMP-9 mRNA. (A) After gel polymerization, culture medium containing 100 μg/ml of anti-MMP-9, or nonimmune IgG was added in the absence of tetracycline (Tet−), and gel contraction was measured. Control IgG (100 μg/ml) was also tested in the presence of tetracycline. Data are expressed as the percent of gel contraction. *Anti-MMP-9 vs. IgG, P < 0.05 (n=3). (B) Gelatin zymography of serum-free medium conditioned 24 h in the absence of tetracycline by cells transfected with siRNA targeting MMP-9 mRNA or scrambled siRNA (5 nM) 3 and 5 days after transfection. (C) Level of collagen gel contraction by cells in Tet+ condition or by cells transfected with siRNA targeting MMP-9 mRNA or scrambled siRNA (5 nM) in Tet− conditions. Data are expressed as percent of gel contraction. *siMMP-9 vs. siSC, P < 0.05 (n=3).
Because it has been reported that MMP-9 is required for SMC-mediated collagen gel contraction [18], we investigated the possibility that MMP-9 may play a different role when expressed at normal or low levels. When anti-MMP-9 (50–200 μg/ml) was added to SMCs expressing only low levels of endogenous MMP-9 (Tet+), collagen gel contraction was significantly inhibited as compared to the IgG control group. Optimal inhibition of gel contraction was obtained with 100 μg/ml of anti-MMP-9 (Fig. 4A). Similarly, treatment with MMP-9 siRNA induced a significant decrease of collagen gel contraction by SMCs cultured in the Tet+ condition (Fig. 4B).
Fig. 4.
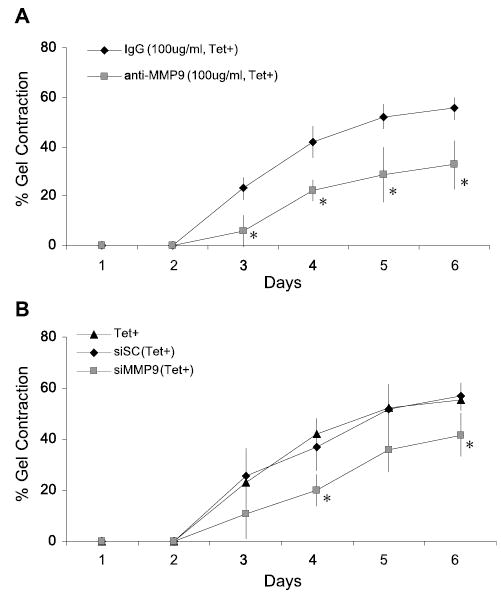
The complete knock-down of MMP-9 inhibits collagen gel contraction by rat SMC(tTA-MMP-9). (A) After gel polymerization, culture medium containing 100 μg/ml of anti-MMP-9 or control IgG was added in the presence of tetracycline (Tet+), and the level of collagen gel contraction was measured. Data are expressed as percent of gel contraction. *anti-MMP-9 vs. IgG, P < 0.05 (n=3). (B) Level of collagen gel contraction by cells transfected with siRNA targeting MMP-9 mRNA or scrambled siRNA (5 nM) or not transfected in the Tet+ condition. Data are expressed as percent of gel contraction. *siMMP-9 vs. siSC, P < 0.05 (n=3).
3.2. Regulation of collagen gel contraction by MMP-9 is dose-dependent
Since MMP-9 seemed to inhibit (Fig. 3) or to stimulate (Fig. 4) collagen gel contraction depending upon its concentration, a dose–response experiment was performed (Fig. 5). Conditioned medium from rat-SMC overexpressing MMP-9 (Tet−) was diluted or concentrated to achieve a wide concentration range of MMP-9 (0.8–300 ng/ml), as estimated by comparative zymography under linear response conditions. The complete MMP-9 depleted conditioned medium (0.0 ng/ml) was obtained from SMC treated both with tetracycline, to repress the expression of recombinant MMP-9, and MMP-9 siRNA, to inhibit the low level of endogenous MMP-9 synthesis. SMCs used for the gel contraction assay were treated to prevent any MMP-9 production (MMP-9 siRNA pretreatment and Tet+ condition during the assay). In these conditions, addition of increasing amounts of recombinant MMP-9 (0–300 ng/ml) resulted in a bell-shaped dose–response curve (Fig. 5A). Collagen gel contraction by SMCs was significantly increased by addition of 0.8 ng/ml MMP-9 compared to the absence of MMP-9 but was inhibited by high concentrations of MMP-9. In addition, control-conditioned medium, obtained from SMC treated with tetracycline, to repress expression of recombinant MMP-9, and with scrambled siRNA (endogenous MMP-9 is not inhibited), did not significantly alter collagen gel contraction compared with conditioned medium obtained from SMCs treated only with tetracycline to repress expression of recombinant MMP-9 (day 6: 56 ± 4% vs. 58 ± 5%, respectively). To evaluate whether the effects on gel contraction observed with conditioned medium was specific to MMP-9 and independent of other factors, MMP-9 was purified from SMC(tTA-MMP9)-conditioned medium. Similar effects on collagen gel contraction were observed with purified MMP-9 at concentrations of 0, 0.8, 50, and 100 ng/ml (Fig. 5B). These results confirm that MMP-9 is responsible for the inhibitory or stimulatory effects on SMC-mediated collagen gel contraction.
Fig. 5.

The regulation of collagen gel contraction by MMP-9 is dose-dependent. (A) Conditioned medium from rat SMC(tTA-MMP-9) over-expressing MMP-9 (Tet−) was diluted or concentrated to achieve a wide range of MMP-9 (0–300 ng/ml), as demonstrated by zymography. Collagen gel contraction by SMC treated to prevent any MMP-9 production (MMP-9 siRNA transfection and Tet+ condition) was then measured in the presence of conditioned medium containing increasing concentration of MMP-9. Data are expressed as percent of gel contraction at day 6. *P < 0.05 (n=3). (B) Collagen gel contraction by SMCs treated to prevent any MMP-9 production (MMP-9 siRNA transfection and Tet+ condition) was measured in presence of purified rat MMP-9 (0, 0.8, 50, and 100 ng/ml). Data are expressed as percent of gel contraction at day 6. *P < 0.05 (n=3).
3.3. BB-94 and TIMP-1 do not reverse the inhibition of gel contraction by MMP-9
The contribution of the proteolytic activity of MMP-9 on gel contraction was investigated by using the broad-spectrum MMPs inhibitor BB-94 and the physiological tissue inhibitor of metalloproteinase-1 (TIMP-1). Fig. 6A demonstrates that BB-94 (5 μM) [19] and TIMP-1 with a ratio TIMP-1/MMP-9 of 1 did not alter the inhibition of SMC-mediated collagen gel contraction induced by MMP-9 overexpression. In addition, BB-94 and TIMP-1 did not alter collagen gel contraction in the control (Tet+) condition. Similar results were observed with higher doses of BB-94 (up to 20 μM) and of TIMP-1 (TIMP-1/MMP-9 ratio up to 50; data not shown).
Fig. 6.

BB-94 and TIMP-1 do not impair the inhibition of collagen gel contraction by MMP-9. (A) After gel polymerization, DMEM with 10% MMP-9-depleted serum, with (Tet+) or without (Tet−) tetracycline, and with BB94 (5 μM) or TIMP-1 (ratio TIMP-1/MMP-9 of 1) or DMSO was added, and the level of collagen gel contraction was measured. Data are expressed as percent of gel contraction. *Tet− vs. Tet+ condition, P < 0.05 (n=3). (B) Effect of metalloproteinase inhibitors (BB-94 and TIMP-1) and MMP-9 antibody on rat-MMP-9 enzymatic activity was investigated using in vitro activity assays, as described in Methods. Results are expressed in percentage of inhibition.
To demonstrate the functionality of the MMP inhibitors as well as to investigate the effect of MMP-9 antibody on MMP-9 activity in our model, activity assays were performed. BB-94 as well as TIMP-1 inhibited the cleavage of the fluorescent substrate by APMA-activated MMP-9, while the MMP-9 antibody did not affect MMP-9 activity (Fig. 6B). No activity was detected when MMP-9 was not activated in vitro with APMA, confirming the absence of an activated form of MMP-9 in our system.
4. Discussion
Arterial wall remodeling is one of the most important factors regulating lumen diameter in early atherosclerosis and after angioplasty [20–23]. MMPs have been studied in relation to nonatherosclerotic arterial geometrical remodeling in animal models. Inhibition of MMPs resulted in impaired constrictive remodeling after balloon angioplasty [6,24,25] and in impaired expansive remodeling after flow enhancement [26].
In this study, we have demonstrated that MMP-9 has a biphasic dose–response effect on SMC-mediated collagen gel contraction in vitro, with low concentrations increasing and high concentrations decreasing contraction. These observations offer an explanation for the apparent discrepancy between our previous observation that MMP-9 enhances positive remodeling in vivo and the observation of Galis et al. [10] showing that MMP-9 mediates constrictive remodeling in vivo. Interestingly, Godin et al. [27] showed that a significant increase in MMP-9 expression preceded positive geometrical remodeling in the mouse flow cessation model, although this was not observed using another mouse strain [10].
Our observations are also of interest because concentrations of MMP-9 that we found to inhibit collagen gel contraction in our model (≥ 100 ng/ml) are observed in the coronary wall with unstable plaque [28] and in human blood under various circumstances including intense atherosclerosis [29]. Thus, it is possible that MMP-9 may mediate the positive arterial remodeling that is observed in conditions in which MMP-9 is present at high concentrations. For example, MMP-9 is observed at higher levels in positively remodeled segments of atherosclerotic arteries than in constrictively remodeled segments [30]. We also showed that when MMP-9 is absent, the ability of SMC to contract collagen gels is impaired. These results confirm and extend previous observations that low amounts of MMP-9 increased the contraction of collagen gels by SMCs as compared to a complete depletion of MMP-9 [18].
Of particular interest is the observation that the proteolytic activity of MMP-9 is not required for the biphasic dose–response effect on collagen contraction. Not only did BB94 and TIMP-1 not reverse the inhibitory effect of high levels of MMP-9, they also did not alter contraction in the presence of endogenous levels of MMP-9 (+Tet conditions). These data suggest that both the stimulatory and the inhibitory properties of MMP-9 on collagen gel contraction do not require proteolytic activity. It has become clear in recent years that MMPs can affect cells by mechanisms other than degradation of extracellular matrix proteins [31]. For example, MMP-2 binds to αvβ3 [32,33] and β1 [33] integrins. The inhibition of contraction by MMP-9 independently of its proteolytic activity observed in our study reinforces the concept that MMPs are much more than simply enzymes involved in the destruction of the ECM.
MMP-9 may modulate SMC contraction of collagen gels independently of its proteolytic function through several mechanisms. First, CD44, a receptor for hyaluronan, plays a role in SMC-mediated collagen gel contraction [18,34]. Yu and Stamenkovic [35] demonstrated that MMP-9 binds to CD44 in vitro. Similarly, Johnson and Galis [18] showed in situ that MMP-9 and CD44 colocalize within the arterial wall. They also demonstrated that CD44 and MMP-9 are necessary for SMC-matrix interactions. Therefore, MMP-9 may act as a bridge between the cell surface and the ECM as suggested by Johnson and Galis [18]. Complete depletion of MMP-9 may inhibit gel contraction, while elevated MMP-9 may saturate the receptor CD44 as well as the pericellular collagen, impairing interactions of CD44, MMP-9, and collagen. Second, the β1 integrins are critical for contraction of a collagen matrix, as blocking antibodies specific for α2β1 prevent contraction in vitro [36–38]. Since MMP-9 may bind to β1 integrin [39], a competition between MMP-9 and collagen for this integrin may occur, leading to reduced collagen gel contraction. Finally, Nassar et al. [40,41] showed that low density lipoprotein-related receptor (LRP) mediates Ca2+ mobilisation and contraction of SMC. Similar results were obtained using human fibroblasts [42]. Since MMP-9 is a ligand for LRP [43], overexpression of MMP-9 may inhibit SMC contraction by blocking LRP-mediated Ca2+ entry.
Our observations demonstrate that while low levels of MMP-9 promote SMC/ECM contraction, high levels inhibit this process. Interestingly, although MMPs are now known to have numerous substrates in addition to ECM molecules [44], these effects on gel contraction appear to be independent of MMP-9 proteolytic activity. Up-regulation of MMP-9 seen after vascular injury may modify the contractile ability of SMCs resulting in positive remodeling, an initial beneficial phase after vascular injury. A better understanding of such a new function of MMP-9 is necessary to develop adequate therapeutic strategies for vascular disease.
Acknowledgments
The authors thank Dr. F. Frankenne and D. Delapierre of the Laboratory of Tumor and Developmental Biology, University of Liège, Sart-Tilman, for their help with the MMP activity assay. Supported by grants from the National Institutes of Health, US Public Health Service (HL18645 and HL30946) and from the Belgian ‘Fonds Scientifique de la Recherche Médicale’ and ‘Fondation L. Fredericq’ (FRSM 3.4566.99).
References
- 1.Pasterkamp G, de Kleijn DP, Borst C. Arterial remodeling in atherosclerosis, restenosis and after alteration of blood flow: potential mechanisms and clinical implications. Cardiovasc Res. 2000;45:843–52. doi: 10.1016/s0008-6363(99)00377-6. [DOI] [PubMed] [Google Scholar]
- 2.Gibbons GH, Dzau VJ. The emerging concept of vascular remodeling. N Engl J Med. 1994;330:1431–8. doi: 10.1056/NEJM199405193302008. [DOI] [PubMed] [Google Scholar]
- 3.Faxon DP, Coats W, Currier J. Remodeling of the coronary artery after vascular injury. Prog Cardiovasc Dis. 1997;40:129–40. doi: 10.1016/s0033-0620(97)80005-9. [DOI] [PubMed] [Google Scholar]
- 4.Schwartz SM, deBlois D, O’Brien ER. The intima. Soil for atherosclerosis and restenosis Circ Res. 1995;77:445–65. doi: 10.1161/01.res.77.3.445. [DOI] [PubMed] [Google Scholar]
- 5.Strauss BH, Chisholm RJ, Keeley FW, Gotlieb AI, Logan RA, Armstrong PW. Extracellular matrix remodeling after balloon angioplasty injury in a rabbit model of restenosis. Circ Res. 1994;75:650–8. doi: 10.1161/01.res.75.4.650. [DOI] [PubMed] [Google Scholar]
- 6.Courtman DW, Franco CD, Meng Q, Bendeck MP. Inward remodeling of the rabbit aorta is blocked by the matrix metalloproteinase inhibitor doxycycline. J Vasc Res. 2004;41:157–65. doi: 10.1159/000077145. [DOI] [PubMed] [Google Scholar]
- 7.Galis ZS, Khatri JJ. Matrix metalloproteinases in vascular remodeling and atherogenesis: the good, the bad, and the ugly. Circ Res. 2002;90:251–62. [PubMed] [Google Scholar]
- 8.Mason DP, Kenagy RD, Hasenstab D, Bowen-Pope DF, Seifert RA, Coats S, et al. Matrix metalloproteinase-9 overexpression enhances vascular smooth muscle cell migration and alters remodeling in the injured rat carotid artery. Circ Res. 1999;85:1179–85. doi: 10.1161/01.res.85.12.1179. [DOI] [PubMed] [Google Scholar]
- 9.Lessner SM, Martinson DE, Galis ZS. Compensatory vascular remodeling during atherosclerotic lesion growth depends on matrix metalloproteinase-9 activity. Arterioscler Thromb Vasc Biol. 2004;24:1–7. doi: 10.1161/01.ATV.0000141840.27300.fd. [DOI] [PubMed] [Google Scholar]
- 10.Galis ZS, Johnson C, Godin D, Magid R, Shipley JM, Senior RM, et al. Targeted disruption of the matrix metalloproteinase-9 gene impairs smooth muscle cell migration and geometrical arterial remodeling. Circ Res. 2002;91:852–9. doi: 10.1161/01.res.0000041036.86977.14. [DOI] [PubMed] [Google Scholar]
- 11.Grinnell F. Fibroblasts, myofibroblasts, and wound contraction. J Cell Biol. 1994;124:401–4. doi: 10.1083/jcb.124.4.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Geary RL, Nikkari ST, Wagner WD, Williams JK, Adams MR, Dean RH. Wound healing: a paradigm for lumen narrowing after arterial reconstruction. J Vasc Surg. 1998;27:96–106. doi: 10.1016/s0741-5214(98)70296-4. [discussion 106–8] [DOI] [PubMed] [Google Scholar]
- 13.Labarca C, Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980;102:344–52. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- 14.Oltenfreiter R, Staelens L, Lejeune A, Dumont F, Frankenne F, Foidart JM, et al. New radioiodinated carboxylic and hydroxamic matrix metalloproteinase inhibitor tracers as potential tumor imaging agents. Nucl Med Biol. 2004;31:459–68. doi: 10.1016/j.nucmedbio.2003.10.013. [DOI] [PubMed] [Google Scholar]
- 15.Deroanne C, Vouret-Craviari V, Wang B, Pouyssegur J. EphrinA1 inactivates integrin-mediated vascular smooth muscle cell spreading via the Rac/PAK pathway. J Cell Sci. 2003;116:1367–76. doi: 10.1242/jcs.00308. [DOI] [PubMed] [Google Scholar]
- 16.Morodomi T, Ogata Y, Sasaguri Y, Morimatsu M, Nagase H. Purification and characterization of matrix metalloproteinase 9 from U937 monocytic leukaemia and HT1080 fibrosarcoma cells. Biochem J. 1992;285:603–11. doi: 10.1042/bj2850603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zempo N, Kenagy RD, Au YP, Bendeck M, Clowes MM, Reidy MA, et al. Matrix metalloproteinases of vascular wall cells are increased in balloon-injured rat carotid artery. J Vasc Surg. 1994;20:209–17. doi: 10.1016/0741-5214(94)90008-6. [DOI] [PubMed] [Google Scholar]
- 18.Johnson C, Galis ZS. Matrix metalloproteinase-2 and -9 differentially regulate smooth muscle cell migration and cell-mediated collagen organization. [see comment]Arterioscler. Thromb Vasc Biol. 2004;24:54–60. doi: 10.1161/01.ATV.0000100402.69997.C3. [DOI] [PubMed] [Google Scholar]
- 19.Davies B, Brown PD, East N, Crimmin MJ, Balkwill FR. A synthetic matrix metalloproteinase inhibitor decreases tumor burden and prolongs survival of mice bearing human ovarian carcinoma xenografts. Cancer Res. 1993;53:2087–91. [PubMed] [Google Scholar]
- 20.Andersen HR, Maeng M, Thorwest M, Falk E. Remodeling rather than neointimal formation explains luminal narrowing after deep vessel wall injury: insights from a porcine coronary (re)stenosis model. [see comment]Circulation. 1996;93:1716–24. doi: 10.1161/01.cir.93.9.1716. [DOI] [PubMed] [Google Scholar]
- 21.Mintz GS, Popma JJ, Pichard AD, Kent KM, Satler LF, Wong C, et al. Arterial remodeling after coronary angioplasty: a serial intravascular ultrasound study. Circulation. 1996;94:35–43. doi: 10.1161/01.cir.94.1.35. [DOI] [PubMed] [Google Scholar]
- 22.Post MJ, de Smet BJ, van der Helm Y, Borst C, Kuntz RE. Arterial remodeling after balloon angioplasty or stenting in an atherosclerotic experimental model. Circulation. 1997;96:996–1003. doi: 10.1161/01.cir.96.3.996. [DOI] [PubMed] [Google Scholar]
- 23.Kimura T, Kaburagi S, Tamura T, Yokoi H, Nakagawa Y, Hamasaki N, et al. Remodeling of human coronary arteries undergoing coronary angioplasty or atherectomy. Circulation. 1997;96:475–83. doi: 10.1161/01.cir.96.2.475. [DOI] [PubMed] [Google Scholar]
- 24.de Smet BJ, de Kleijn D, Hanemaaijer R, Verheijen JH, Robertus L, van Der Helm YJ, et al. Metalloproteinase inhibition reduces constrictive arterial remodeling after balloon angioplasty: a study in the atherosclerotic Yucatan micropig. Circulation. 2000;101:2962–7. doi: 10.1161/01.cir.101.25.2962. [DOI] [PubMed] [Google Scholar]
- 25.Sierevogel MJ, Pasterkamp G, Velema E, De Kleijn DPV, De Smet PPT, De Jaegere PPT, et al. Oral matrix metalloproteinase inhibition blocks constrictive arterial remodeling following ballon dilatation in the pig. Circulation. 2001;103:302–7. doi: 10.1161/01.cir.103.2.302. [DOI] [PubMed] [Google Scholar]
- 26.Karwowski JK, Markezich A, Whitson J, Abbruzzese TA, Zarins CK, Dalman RL. Dose-dependent limitation of arterial enlargement by the matrix metalloproteinase inhibitor RS-113,456. J Surg Res. 1999;87:122–9. doi: 10.1006/jsre.1999.5707. [DOI] [PubMed] [Google Scholar]
- 27.Godin D, Ivan E, Johnson C, Magid R, Galis ZS. Remodeling of carotid artery is associated with increased expression of matrix metalloproteinases in mouse blood flow cessation model. Circulation. 2000;102:2861–6. doi: 10.1161/01.cir.102.23.2861. [DOI] [PubMed] [Google Scholar]
- 28.Loftus IM, Goodall S, Crowther M, Jones L, Bell PR, Naylor AR, et al. Increased MMP-9 activity in acute carotid plaques: therapeutic avenues to prevent stroke. Ann NY Acad Sci. 1999;878:551–4. doi: 10.1111/j.1749-6632.1999.tb07724.x. [DOI] [PubMed] [Google Scholar]
- 29.Dandona P, Aljada A, Mohanty P, Ghanim H, Bandyopadhyay A, Chaudhuri A. Insulin suppresses plasma concentration of vascular endothelial growth factor and matrix metalloproteinase-9. Diabetes Care. 2003;26:3310–4. doi: 10.2337/diacare.26.12.3310. [DOI] [PubMed] [Google Scholar]
- 30.Pasterkamp G, Schoneveld AH, Hijnen DJ, de Kleijn DP, Teepen H, van der Wal AC, et al. Atherosclerotic arterial remodeling and the localization of macrophages and matrix metalloproteases 1, 2 and 9 in the human coronary artery. Atherosclerosis. 2000;150:245–53. doi: 10.1016/s0021-9150(99)00371-8. [DOI] [PubMed] [Google Scholar]
- 31.Sternlicht MD, Werb Z. How matrix metalloproteinases regulate cell behavior. Annu Rev Cell Dev Biol. 2001;17:463–516. doi: 10.1146/annurev.cellbio.17.1.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brooks PC, Stromblad S, Sanders LC, von Schalscha TL, Aimes RT, Stetler-Stevenson WG, et al. Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell. 1996;85:683–93. doi: 10.1016/s0092-8674(00)81235-0. [DOI] [PubMed] [Google Scholar]
- 33.Levkau B, Kenagy RD, Karsan A, Weitkamp B, Clowes AW, Ross R, et al. Activation of metalloproteinases and their association with integrins: an auxiliary apoptotic pathway in human endothelial cells. Cell Death Differ. 2002;9:1360–7. doi: 10.1038/sj.cdd.4401106. [DOI] [PubMed] [Google Scholar]
- 34.Travis JA, Hughes MG, Wong JM, Wagner WD, Geary RL. Hyaluronan enhances contraction of collagen by smooth muscle cells and adventitial fibroblasts: role of CD44 and implications for constrictive remodeling. [see comment]Circ Res. 2001;88:77–83. doi: 10.1161/01.res.88.1.77. [DOI] [PubMed] [Google Scholar]
- 35.Yu Q, Stamenkovic I. Localization of matrix metalloproteinase 9 to the cell surface provides a mechanism for CD44-mediated tumor invasion. Genes Dev. 1999;13:35–48. doi: 10.1101/gad.13.1.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Skinner MP, Raines EW, Ross R. Dynamic expression of alpha 1 beta 1 and alpha 2 beta 1 integrin receptors by human vascular smooth muscle cells. Alpha 2 beta 1 integrin is required for chemotaxis across type I collagen-coated membranes. Am J Pathol. 1994;145:1070–81. [PMC free article] [PubMed] [Google Scholar]
- 37.Lee RT, Berditchevski F, Cheng GC, Hemler ME. Integrin-mediated collagen matrix reorganization by cultured human vascular smooth muscle cells. Circ Res. 1995;76:209–14. doi: 10.1161/01.res.76.2.209. [DOI] [PubMed] [Google Scholar]
- 38.Schiro JA, Chan BM, Roswit WT, Kassner PD, Pentland AP, Hemler ME, et al. Integrin alpha 2 beta 1 (VLA-2) mediates reorganization and contraction of collagen matrices by human cells. Cell. 1991;67:403–10. doi: 10.1016/0092-8674(91)90191-z. [DOI] [PubMed] [Google Scholar]
- 39.Partridge CA, Phillips PG, Niedbala MJ, Jeffrey JJ. Localization and activation of type IV collagenase/gelatinase at endothelial focal contacts. Am J Physiol. 1997;272:L813–22. doi: 10.1152/ajplung.1997.272.5.L813. [DOI] [PubMed] [Google Scholar]
- 40.Nassar T, Haj-Yehia A, Akkawi S, Kuo A, Bdeir K, Mazar A, et al. Binding of urokinase to low density lipoprotein-related receptor (LRP) regulates vascular smooth muscle cell contraction. J Biol Chem. 2002;277:40499–504. doi: 10.1074/jbc.M207172200. [DOI] [PubMed] [Google Scholar]
- 41.Nassar T, Akkawi S, Bar-Shavit R, Haj-Yehia A, Bdeir K, Al-Mehdi AB, et al. Human alpha-defensin regulates smooth muscle cell contraction: a role for low-density lipoprotein receptor-related protein/alpha 2-macroglobulin receptor. Blood. 2002;100:4026–32. doi: 10.1182/blood-2002-04-1080. [DOI] [PubMed] [Google Scholar]
- 42.Takayama Y, Takahashi H, Mizumachi K, Takezawa T. Low density lipoprotein receptor-related protein (LRP) is required for lactoferrin-enhanced collagen gel contractile activity of human fibroblasts. J Biol Chem. 2003;278:22112–8. doi: 10.1074/jbc.M300894200. [DOI] [PubMed] [Google Scholar]
- 43.Hahn-Dantona E, Ruiz JF, Bornstein P, Strickland DK. The low density lipoprotein receptor-related protein modulates levels of matrix metalloproteinase 9 (MMP-9) by mediating its cellular catabolism. J Biol Chem. 2001;276:15498–503. doi: 10.1074/jbc.M100121200. [DOI] [PubMed] [Google Scholar]
- 44.Somerville RP, Oblander SA, Apte SS. Matrix metalloproteinases: old dogs with new tricks. Genome Biol. 2003;4:216. doi: 10.1186/gb-2003-4-6-216. [DOI] [PMC free article] [PubMed] [Google Scholar]


