Potassium channels play a major role in the immediate and long term regulation of vascular smooth muscle function.1 The activity of these ion channels determines and regulates cell membrane potential, which, in turn, regulates the open state probability of voltage-gated Ca2+ channels, Ca2+ influx, and intracellular Ca2+. The concentration of intracellular Ca2+ not only regulates the immediate contractile responses of smooth muscle cells (ie, vascular tone),1 but also the long term responses of these cells through control of gene expression.2 By their effect on membrane potential, K+ channels also establish the electrochemical gradient that determines the movement of other ions across the plasma membrane. In addition, potassium channels participate significantly in cell volume regulation.3
Over the past two decades it has become apparent that K+ channels also play an important role in cell proliferation.4–8 In vascular smooth muscle cells, investigators have identified increased expression of intermediate conductance, Ca2+-activated K+ (IKCa) channels (KCa3.1, locus: KCNN4) associated with proliferation,4 and recent studies have shown that selective inhibition of these channels prevents vascular smooth muscle proliferation associated with injury-induced restenosis.9 The study by Miguel-Velado et al in this issue of Circulation Research10 confirms an important role for K+ channels in vascular smooth muscle cell proliferation. The authors extend this area by showing that Kv3.4 channel (locus: KCNC4) expression is increased in proliferating smooth muscle cells from human uterine artery and that blockade of these channels inhibits proliferation. These KV channels also have been implicated in proliferation of an oral squamous cell carcinoma,11,12 and their expression is increased in growth cones of retinal ganglion cells.13 Although Kv3.4 channels, and other members of the voltage-gated K+ channel superfamily of K+ channels such as Kv1.3,5,8 Kv10.1, and Kv11.1,5 have been implicated in proliferation of other cell types, the study by Miguel-Velado and associates10 is the first to suggest that the Kv3 family participates in proliferation of smooth muscle cells. This finding is significant for several reasons. First, it suggests that there may be regional or cell-specific differences in the K+ channels that are used during smooth muscle proliferation. Such differences offer the potential for development of regional or cell-specific inhibitors of K+ channels, or the signaling pathways associated with their upregulation, that might be used to control pathological proliferation of vascular smooth muscle cells associated with diseases such as atherosclerosis, hypertension, and cancer. Second, the study by Miguel-Velado et al10 adds to the large body of evidence for a crucial role of K+ channels in cell proliferation. Although there remains little doubt that increased K+ channel expression and function are necessary for cells to proliferate, the mechanistic role for K+ channels in this process remains unclear (see below). By having a new target K+ channel in smooth muscle, it may be possible, through comparative studies using genomic and proteomic approaches, to define commonalities among different K+ channels and proliferation in one cell type. Finally, the study by Miguel-Velado and colleagues10 reinforces the notion that proliferating smooth muscle cells in culture are a poor model system in which to study normal ion channel expression and physiological function.
What remains to be established in smooth muscle and in other cells5 is the mechanistic link among increased K+ channel expression, K+ channel function, and proliferation. Several hypotheses have arisen based on the known functions of K+ channels including effects on membrane potential and calcium signaling, effects on cell volume regulation, and effects related to formation of signaling complexes (Figure).4–6 Studies from lymphocytes and tumor cells suggest that progress into G1 of the cell cycle requires K+ channel–induced membrane hyperpolarization.8 Hyperpolarization does not appear to account for the impact of Kv3.4 channel expression in the study by Miguel-Velado et al,10 because proliferating smooth muscle cells were found to be depolarized compared with freshly isolated cells. However, transient hyperpolarization associated with entrance into G1 was not excluded by Miguel-Velado et al,10 as the cells studied were not synchronized and the cell cycle state in which membrane potential was measured was not assessed. Although not established by the present study, the rapid voltage-dependent inactivation of Kv3.4 channels might actually contribute to the depolarized phenotype in the proliferating cells. In smooth muscle cells from pulmonary arteries, inhibition and decreased expression of other KV channels results in membrane depolarization, elevated intracellular Ca2+, and increased proliferation.14 This mechanism also does not appear to be a viable alternative in uterine artery cells, because Kv3.4 channel blockade inhibited, rather than enhanced, proliferation. Decreased K+ channel function in pulmonary smooth muscle cells limits the ability of the cells to reduce cell volume, an effect that inhibits apoptosis, amplifying cell proliferation.14 Reduced programmed cell death does not appear to play a role in uterine artery cell proliferation, because apoptosis was similar in the presence or absence of a Kv3.4 channel blocker. Although it is intriguing to speculate on the possible role of Kv3.4 channels in formation of signaling complexes important for proliferation,5 it is difficult to understand how Kv3.4 channel blockers might disrupt such complexes to inhibit proliferation as described in the study by Miguel-Valedo et al.10 Thus, the precise mechanism by which Kv3.4 channel expression and function promotes proliferation of uterine artery smooth muscle cells in culture remains to be established but provides fertile ground for future investigation.
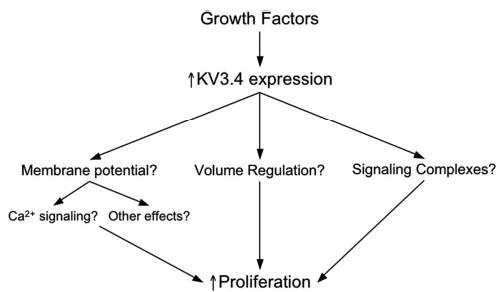
Mechanisms by which increased Kv3.4 channel expression might increase cell proliferation. Growth factors, such as those present in serum, trigger expression of Kv3.4 channels. The increased expression of Kv3.4 channels then has the potential to impact cell membrane potential, volume regulation, and formation of proliferation-related macromolecular signaling complexes. Changes in membrane potential, or its regulation, could then affect cell calcium signaling, or other membrane potential-sensitive mechanisms. Changes in cell volume, or its regulation, could alter concentrations or associations of signaling molecules, or produce other effects that might enhance proliferation. Finally, Kv.3.4 channels might interact with other signaling proteins involved in proliferation to form unique signaling complexes and in this way promote cell proliferation.
Acknowledgments
This work was supported by Public Health Service grant HL32469.
Footnotes
The opinions expressed in this editorial are not necessarily those of the editors or of the American Heart Association.
References
- 1.Jackson WF. Potassium channels in the peripheral microcirculation. Microcirculation. 2005;12(1):113–127. doi: 10.1080/10739680590896072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cartin L, Lounsbury KM, Nelson MT. Coupling of Ca(2+) to CREB activation and gene expression in intact cerebral arteries from mouse: roles of ryanodine receptors and voltage-dependent Ca(2+) channels. Circ Res. 2000;86(7):760–767. doi: 10.1161/01.res.86.7.760. [DOI] [PubMed] [Google Scholar]
- 3.Tang XD, Santarelli LC, Heinemann SH, Hoshi T. Metabolic regulation of potassium channels. Annu Rev Physiol. 2004;66:131–159. doi: 10.1146/annurev.physiol.66.041002.142720. [DOI] [PubMed] [Google Scholar]
- 4.Neylon CB. Potassium channels and vascular proliferation. Vascul Pharmacol. 2002;38(1):35–41. doi: 10.1016/s1537-1891(02)00124-6. [DOI] [PubMed] [Google Scholar]
- 5.Pardo LA. Voltage-gated potassium channels in cell proliferation. Physiology (Bethesda) 2004;19:285–292. doi: 10.1152/physiol.00011.2004. [DOI] [PubMed] [Google Scholar]
- 6.Wang Z. Roles of K+ channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448(3):274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- 7.DeCoursey TE, Chandy KG, Gupta S, Cahalan MD. Voltage-gated K+ channels in human T lymphocytes: a role in mitogenesis? Nature. 1984;307(5950):465–468. doi: 10.1038/307465a0. [DOI] [PubMed] [Google Scholar]
- 8.Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996;154(2):91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- 9.Kohler R, Wulff H, Eichler I, Kneifel M, Neumann D, Knorr A, Grgic I, Kampfe D, Si H, Wibawa J, Real R, Borner K, Brakemeier S, Orzechowski HD, Reusch HP, Paul M, Chandy KG, Hoyer J. Blockade of the intermediate-conductance calcium-activated potassium channel as a new therapeutic strategy for restenosis. Circulation. 2003;108(9):1119–1125. doi: 10.1161/01.CIR.0000086464.04719.DD. [DOI] [PubMed] [Google Scholar]
- 10.Miguel-Velado E, Moreno-Dominguez A, Colinas O, Cidad P, Heras M, Perez-Garcia MT, Lopez-Lopez JR. Contribution of Kv channels to phenotypic remodelling of human uterine artery smooth muscle cells. Circ Res. 2005;97:1280–1287. doi: 10.1161/01.RES.0000194322.91255.13. [DOI] [PubMed] [Google Scholar]
- 11.Chang KW, Yuan TC, Fang KP, Yang FS, Liu CJ, Chang CS, Lin SC. The increase of voltage-gated potassium channel Kv. 3.4 mRNA expression in oral squamous cell carcinoma. J Oral Pathol Med. 2003;32(10):606–611. doi: 10.1034/j.1600-0714.2003.00197.x. [DOI] [PubMed] [Google Scholar]
- 12.Lew TS, Chang CS, Fang KP, Chen CY, Chen CH, Lin SC. The involvement of K(v)3.4 voltage-gated potassium channel in the growth of an oral squamous cell carcinoma cell line. J Oral Pathol Med. 2004;33(9):543–549. doi: 10.1111/j.1600-0714.2004.00236.x. [DOI] [PubMed] [Google Scholar]
- 13.Pollock NS, Ferguson SC, McFarlane S. Expression of voltage-dependent potassium channels in the developing visual system of Xenopus laevis. J Comp Neurol. 2002;452(4):381–391. doi: 10.1002/cne.10401. [DOI] [PubMed] [Google Scholar]
- 14.Mandegar M, Fung YC, Huang W, Remillard CV, Rubin LJ, Yuan JX. Cellular and molecular mechanisms of pulmonary vascular remodeling: role in the development of pulmonary hypertension. Microvasc Res. 2004;68(2):75–103. doi: 10.1016/j.mvr.2004.06.001. [DOI] [PubMed] [Google Scholar]


