Abstract
Purpose
To evaluate the safety and efficiency of feline immunodeficiency virus (FIV) vectors for gene delivery into the mammalian retina.
Methods
A first-generation FIV vector was constructed and administered into rabbit eyes at two different concentrations by intravitreal or subretinal routes. A second-generation FIV vector was also constructed and administered subretinally into both rabbit and rat eyes at the same concentration. After vector administration, eyes were monitored using slit-lamp biomicroscopy, indirect ophthalmoscopy, fundus photography, and electroretinogram. After the rabbits were killed, eye tissues were processed for light microscopy and immunohistochemical analysis.
Results
Administration of both first- and second-generation FIV vectors produced transient vitritis and/or papillitis in rabbits, without other pathologic abnormalities. Retinal pigment epithelium (RPE) cells were the predominant cell type transduced in rabbit eyes, but ganglion cells and Müller cells were also transduced. Transduction was confined to the retinal bleb area. The second-generation FIV vector transduced RPE cells much more efficiently than the first-generation vector (95% vs. 4.5%, respectively; P = 0.0015) in rabbit eyes. In contrast, no toxicity was evident over a 24- to 25-month follow-up period after injection of the second-generation FIV vector into rat eyes. Tropism in the rat eye was similar, including RPE and ganglion cells, and the RPE transduction rate was also high (50%). Transgene expression was persistent in both species over the duration of the experiment.
Conclusion
Second-generation FIV vectors can efficiently transfer genes into RPE cells with resulting long-term expression, properties potentially valuable to gene therapy approaches to some retinal diseases.
Keywords: feline immunodeficiency virus, intravitreal drug delivery, lentivirus vectors, rabbit, rat, retinal gene therapy
This study provides data on feline immunodeficiency virus (FIV) vector transduction of retinal cells in two commonly used species of experimental animals as well as the longest follow-up yet available of transgene expression and potential toxicity. The second-generation FIV vector demonstrated highly effective retinal pigment epithelium transduction and stable transgene expression in both rabbit and rat eyes, indicating its promising clinical application.
The introduction of a neurotrophic protein or other therapeutic gene into the retina is a promising strategy for treatment of refractory retinal conditions, including age-related macular degeneration and other degenerative retinal diseases.1 Several viral vectors have been successfully used to transfer genes into postmitotic retinal cells.1–10 Among these, adenovirus, adeno-associated virus, and lentivirus vectors have been found to efficiently deliver genes of interest into retinal cells in vitro and in vivo.3,8,11–14 Lentiviruses can efficiently infect nonmitotic cells15 and permanently integrate their reverse-transcribed DNA into the host cell genome, a major potential advantage over adeno-associated virus and adenovirus for long-term gene expression. Other advantages of the lentivirus systems include a respectable cloning capacity (close to 10 kilobases), which is larger than that of adeno-associated virus. In addition, lentivirus vectors have the ability to mediate high levels of persistent transgene expression in vivo and induce little host immune response.8,13,14
So far, most gene transfer approaches involving lentivirus vectors have been performed with HIV-based vectors.8,13,14,16,17 Concerns with HIV-based vectors have included the risk of generating replication-competent recombinant virus during production, risks of recombination and vector mobilization in HIV-infected individuals, and potential exposure to HIV proteins, which may have toxic effects18 and/or produce seroconversion.
Feline immunodeficiency virus (FIV) is another member of the lentivirus family, which is antigenically and genetically19 distinct from HIV. FIV has not been reported to cause human infection or disease,20 although veterinary exposure must occur frequently. FIV vectors pseudotyped with VSV-G have shown the ability to efficiently infect nondividing cells, both in vitro and in vivo, and to produce persistent transgene expression.21,22 Recently, initial findings concerning the use of FIV vectors in treating eye diseases have been reported.23–27 In the current study, the safety, transduction efficiency, and stability of transgene expression from FIV vectors over a 2-year follow-up period are described, and the cell tropism of first- and second-generation FIV vectors examined in two experimental animal species is reported. FIV vectors show considerable promise for retinal gene therapy.
Materials and Methods
FIV Vectors
The first-generation vector system (packaging plasmid CF1Denv and vector pCTRZLb) has been previously described.22 Briefly, pseudotyped vector for transduction was prepared by calcium phosphate transfection of 293T fibroblasts with plasmids expressing vesicular stomatitis virus glycoprotein G (pHCMVGV), CF1Denv, and pCTRZLb. Supernatants were collected 48 to 96 hours after transfection, cleared by low-speed centrifugation, filtered, divided into aliquots, and stored at −80°C until use. Vectors were titrated on CRFK and HT1080 cells, and staining foci were scored after X-gal staining, with values expressed as transducing units per milliliter (TU/mL).
The second-generation FIV vector system (pC34N/pVC-LacZwP) has also been previously described.28 Differences from the first-generation system include deletion of the principal packaging domain, a larger deletion in the env gene, use of the SV40 polyadenylation signal in packaging construct pC34N, and a reduction of retained gag gene sequences (800 base pairs less) and introduction of the woodchuck hepatitis B virus posttranscriptional regulatory element downstream of the lacZ gene to enhance transgene expression in the vector construct pVC-LacZwP.28 These changes facilitated a 40- to 200-fold increase in titer after transfection, making concentrated preparations of >1 × 108 TU/mL routinely achievable.
Animal Studies
Thirty-three pigmented adult New Zealand Red rabbits (weighing 1.9–2.6 kg at the beginning of the experiment) were used in the pCTRZLb vector study in accordance with the guidelines of the University of California, San Diego, Animal Care and Animal Subjects Programs and the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research. Seventeen animals were used for evaluation of the lower-concentration FIV vector (2 × 105 TU/mL), and the remaining 16 were used for evaluation of the higher-concentration FIV vector (2.4 × 107 TU/mL). Animals injected with the lower concentration were evaluated at weeks 1, 3, 6, 12, and 26 after inoculation, and those injected with the higher concentration were evaluated at 1, 2, and 4 weeks after inoculation. Eyes were dilated with one drop each of tropicamide (Mydriacyl 1%; Alcon, Puerto Rico) and phenylephrine hydrochloride 2.5% (Bausch and Lomb, Tampa, FL) before anesthesia. After anesthesia and subsequent paracentesis through the cornea limbus,29 vector was injected into the vitreous body or subretinal space. Intravitreal and subretinal injections of medium (Dulbecco modified Eagle medium) were also performed in two eyes at each time point as controls. For intravitreal injection, a 30-gauge needle was introduced 2 mm behind the limbus, and 50 μL of vector was injected from the 500-μL syringe into the vitreous cavity between the lens and the retina. One eye at each time point in the low-dose group received a 100-μL intravitreal vector injection. For subretinal injection, the viral vector solution was loaded into a 500-μL sterilized Hamilton syringe, and 40 μL was injected into the subretinal space using a 20-gauge needle tapered to 41 gauge (Storz Ophthamics, Inc., St Louis, MO). The injections were made at the retina below the optic nerve head viewed through a surgical microscope. Dome-shaped neurosensory retinal detachments were produced, confirming the location of the virus inoculation between sensory retina and retinal pigment epithelium (RPE). The location and size of the retinal bleb was documented in relation to the optic nerve head and in comparison with the diameter of the optic nerve head. If significant vitreous or subretinal hemorrhage occurred, the eyes were excluded from the study. After the procedure, antibiotic eye ointment (tobramycin) was applied topically.
In the experiments with pVC-LacZwP, 8 pigmented rabbits and 13 Brown Norway rats received only sub-retinal injections. Right eyes received viral vector injection, and left eyes received an equivalent volume of diluent (phosphate-buffered saline [PBS]). The rabbits were killed and evaluated at 1 (3 rabbits), 2 (3 rabbits), and 4 (2 rabbits) weeks; the rats were killed and evaluated at 1 week (3 rats), 2 weeks (3 rats), 4 weeks (3 rats), 18 months (2 rats), and 24.25 months (2 rats) after viral vector subretinal injections. Vector (40 μL of 1.5 × 107 TU/mL pVC-LacZwP FIV) was subretinally injected into rabbit eyes, while 5 μL was injected into rat eyes. The subretinal injection procedure was the same as described above for rabbits. For rats, an entry hole was made 2 mm behind the limbus with a 27-gauge needle, diluted vector was loaded into a 250-μL sterilized Hamilton syringe on the Hamilton repeating pipette dispenser system, and 5 μL was injected into the subretinal space using a 20 × 41-gauge needle (Storz Ophthamics, Inc.) on an extended tubing apparatus. On this pipette dispenser system, each actuation of the dispense button delivers exactly 1/50th of the total capacity of the syringe to which it is connected. Immediately after the injection, the location of retinal detachment was documented in relation to the 12-o’clock position on the cornea and the optic nerve head. At the same time, the area of the detachment was also documented as a fraction of the whole retina viewed with the surgical microscope. After the injections, anterior segment congestion, lens clarity, vitreous clarity,29 vitreous or retinal hemorrhage, and fundus were assessed and documented by slit-lamp biomicroscopy and indirect ophthalmoscopy at day 1, day 3, week 1, week 2, week 3, week 4, and then every other week until the animals were killed. Fundus photographs were taken to illustrate normal and abnormal fundi at different time points. Full-field scotopic electroretinograms were obtained before the animals were killed as described previously.30,31 The animals were killed at scheduled time points after viral vector injection, and the globes were enucleated for histologic processing and immunohistochemical analysis for LacZ detection.
Immunohistochemical Analysis and Pathology Study
After marking the 12-o’clock position of the globe by suture and globe enucleation, one half of the cornea and the whole lens were removed, and the eye was immediately fixed in 0.5% glutaraldehyde in PBS (30 minutes at 4°C). For LacZ staining, eyecups were washed with cold PBS and incubated at 37°C for 4 hours in a solution of 1 mg/mL 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (X-Gal; Sigma Chemical Company, St. Louis, MO), 5 mmol/L K4Fe(CN)6, 5 mmol/L K4Fe(CN)6–3H2O, 2 mmol/L MgCl2 in PBS. X-Gal–reacted eyecups were washed in PBS, gross inspection was performed, and the findings were documented on our standardized gross inspection sheet. Photographs were also taken of selected eyes. The globe was then postfixed for 2 to 10 hours in a solution containing 2% paraformaldehyde/2% glutaraldehyde in PBS pH 7.2 or in 4% paraformaldehyde in PBS pH 7.0 at 4°C. If the eye received an intravitreal injection, the eyecup was cut into two halves through the 12-o’clock position and through the optic nerve head. If the eye had subretinal injection, the eyecup was cut into two halves through the middle of the bleb and through the optic nerve head. After a brief rinse with PBS three times, one half was infiltrated in 30% sucrose overnight and then frozen in OCT compound; the other half underwent graded dehydration and routine paraffin infiltration.
The cells stained blue were counted, and the blue intensity was graded from neutral red–counterstained serial cryosections (6 μm). At least 10 sections, 100 μm apart from each other, were used to determine the percentage of cell staining. Each section used for cell counting contained a full-length retina that began from the superior ora, crossed the posterior pole, and ended at the inferior ora. The ratio of stained cells to non-stained cells was obtained from averaging the number of RPE cells and ganglion cells that were easily identified according to their location and morphology. For intravitreally injected eyes, the cell count was performed from ora to ora; for subretinally injected eyes, the cell count was performed in the retinal bleb area that was documented previously. The intensity of blue staining of the cells was graded as 3+ (dark blue staining), 2+ (moderate blue staining), or 1+ (weak blue staining) against a control sample under ×400 magnifications. Each section was assigned one score for each cell type; averaging the multiple counted sections yielded an average score for each cell type in each eye.
Retinal toxicity and vitreous inflammation were evaluated from paraffin sections stained with hematoxylin–eosin or from adjacent cryosections with hematoxylin–eosin staining. Vitreous inflammation was measured by counting the number of inflammatory cells in the vitreous cavity from the sections used for stained retinal cell counts under ×400 magnification. Inflammatory cells were morphologically identified as macrophage or mononuclear cells, lymphocytes, and granulocytes including neutrophils, eosinophils, and basophils. For each eye, counts of multiple sections were averaged to obtain one number.
Results
Clinical Observations
No adverse impact on general health attributable to vector injection or any external eye abnormality was observed in any animal during the course of these experiments. In the group receiving the low-dose first-generation vector (pCTRZLb), indirect ophthalmoscopy 1 week after inoculation revealed that the retinal bleb from the subretinal injection had subsided without obvious chorioretinal scar or atrophy. A demarcation line was also observed in some control eyes. One week after intravitreal injection, a mild localized vitreous haze was noted when compared with the control eyes. By 3 weeks after inoculation, 4 eyes that received 100 μL and 1 eye that received 40 μL had a variable hazy vitreous with white precipitates that was accompanied by hyperemia and mild swelling of the optic nerve head. The precipitates and optic nerve changes persisted for up to 4 weeks before resolution. By 8 weeks after inoculation, fundus examination results were unremarkable. Similar changes were noted in animals receiving high-dose pCTRZLb: a localized mild vitreous haze was found in 7 of 20 eyes 1 week after inoculation. Of these seven animals (seven eyes), three (three eyes) were killed 1 and 2 weeks after inoculation, and the remaining four eyes had variable white precipitates until 4 weeks after inoculation (the last time point for this substudy).
In the rabbit eyes that received subretinal injections of the second-generation FIV vector (pVC-LacZwP), variable white precipitates under the retina and in the vitreous around the bleb appeared 3 days after inoculation and then decreased over time. Subretinal white precipitates disappeared around 1 week after injection, but the precipitates in the vitreous did not completely disappear in all eyes before the end point of this experiment (4 weeks after inoculation). One of these eyes developed optic nerve head hyperemia and swelling (Fig. 1; Table 1). Findings for all the left eyes that received PBS injections were unremarkable. Electroretinogram results are shown in Table 2. There was no difference in the parameters between vector and control eyes except that the b-wave implicit was shorter in the vector-injected eyes than in the control-injected eyes (P = 0.01, paired t-test).
Fig. 1.
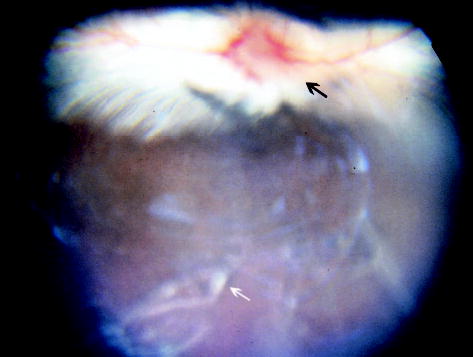
Fundus photograph 4 weeks after subretinal injection of the second-generation feline immunodeficiency virus vector showing white precipitates (white arrow) in the vitreous and hyperemia of the optic nerve head (black arrow).
Table 1.
Inflammation Effects of First- and Second-Generation FIV Vectors in Rabbit Eyes
| No. of Eyes
|
|||
|---|---|---|---|
| No. of Eyes, Vector | Injection Method/Dose | Vitreous Haze With Precipitates | Optic Nerve Hyperemia or Swelling |
| Low-dose first-generation FIV vector at week 3 | |||
| 8 | Control | 0* | 0* |
| 10 | Subret./40 μL | 1* | 1* |
| 5 | Intravit./50 μL | 0* | 0* |
| 5 | Intravit./100 μL* | 4* | 4* |
| High-dose first-generation FIV vector at week 4 | |||
| 2 | Control | 0† | 0† |
| 4 | Subret./40 μL | 1† | 1† |
| 4 | Intravit./50 μL | 3† | 3† |
| Second-generation FIV vector at week 2 | |||
| 5 | Subret./40 μL | 4‡ | 1§ |
| 5 | Control | 0‡ | 0§ |
Intravit./100 μL vs. control (P = 0.007), subret./40 μL (P = 0.017), and intravit./50 μL (P = 0.048).
Intravit./50 μL (100%) vs. subret./40 μL (25%) vs. control (0).
Subret./40 μL vs. control (P = 0.048).
Subret./40 μL vs. control (P = NS).
P values derived from Fisher exact test.
FIV, feline immunodeficiency virus; subret., subretinal; intravit., intravitreal; NS, not significant.
Table 2.
ERGs for Rabbits and Rats That Received pVC-LacZwP in Right Eyes and PBS in Left Eyes
| Species | No. of Animals | Eye | a-it | a-amp | b-it | p to p |
|---|---|---|---|---|---|---|
| Rabbit | 8 | OD | 16 ± 0 | 9.82 ± 5.5 | 57.5 ± 14.3 | 122 ± 35.2 |
| OS | 16 ± 0 | 9.4 ± 6 | 66.5 ± 14.6 | 113.2 ± 20.3 | ||
| P | >0.05 | >0.05 | 0.01 | >0.05 | ||
| Rat | 13 | OD | 24.4 ± 4 | 35.2 ± 36.3 | 64.3 ± 19.7 | 110.4 ± 46.1 |
| OS | 24.1 ± 4.2 | 39.2 ± 38.6 | 56.9 ± 18.4 | 120.8 ± 66.2 | ||
| P | >0.05 | >0.05 | >0.05 | >0.05 |
Data are mean ± SD. P values derived from paired t-test.
ERG, electroretinogram; PBS, phosphate-buffered saline; a-it, a-wave implicit (ms); a-amp, a-wave amplitude (μV); b-it, b-wave implicit (ms); p to p, measured between the trough of a wave to the peak of b wave (μV).
In the rat eyes that received subretinal injections of pVC-LacZwP, no abnormalities were observed throughout the entire experiment (24 months), compared with the fellow eyes that received PBS injection. Of 13 rats, 9 were killed and evaluated between weeks 1 and 4; the remaining 4 rats were evaluated 18 and 24 months after inoculation. The elevated bleb disappeared on the third day with clear vitreous and normal retina. Electroretinogram results for all the eyes were normal, and there were no differences in other parameters between eyes injected with vector and control eyes (Table 2).
Retinal Cell Transduction and Transgene Expression
In the group receiving the low-dose first-generation vector, transgene expression could be detected at 1, 6, 12, and 26 weeks after inoculation in RPE, ganglion, or Müller cells in only 4 of 24 vector-injected eyes, all of which received subretinal injections. No transgene expression could be seen in eyes that received intravitreal injection of vector or control eyes that received medium. Only scattered RPE cells (≈2%) within the subretinal bleb expressed LacZ (Fig. 2), and the reporter transgene was also expressed by small numbers of ganglion and Müller cells (<1%) near the bleb area (unstained Müller cells are difficult to identify, and the transduction rate was estimated based on the number of blue-stained cells, with their frequency compared with that of ganglion cells). Transduced cells displayed normal morphology, including those from eyes enucleated 26 weeks after inoculation. In the group that received the higher dose of pCTRZLb, 5 of 20 eyes injected with vector had transgene expression in some retinal cell types; only eyes that received subretinal injection (5 of 10) had transgene expression. The transduction rate for RPE cells was improved compared with that for eyes receiving the lower dose of this vector (4.5% vs. 2%, respectively; P = 0.0486, Wilcoxon rank sum test), but this rate was still low, with only scattered expressing cells observed.
Fig. 2.
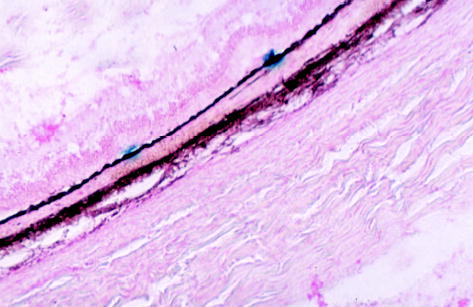
Microscopic photograph of a rabbit eye 26 weeks after subretinal injection of the first-generation feline immunodeficiency virus vector showing two groups of retinal pigment epithelium cells stained blue with normal morphology and normal retina (original magnification, ×62.5; cryosection, 10 μm; neutral red staining).
In both rabbit and rat eyes that received subretinal injections of the second-generation vector pVC-Lac-ZwP, 100% of the eyes had transgene expression in retinal cells within the retinal bleb (Figs. 3 and 4). In both species, RPE cells were the predominant cell type transduced, displaying intense blue staining (grade 3) up to 24 months after inoculation. The intensity of the transgene expression and the percentage of RPE cell transduction did not diminish over time. The average transduction rate of RPE cells ± SD for rabbits was 95% ± 5.3% and 95% ± 4.5% at 2 and 4 weeks after inoculation, respectively (P > 0.05, Student’s t-test; Fig. 5). For rats, the transduction rate of RPE cells ± SD was 47% ± 20% and 50% ± 16% at 2 weeks and 24.25 months, respectively (P > 0.05, Student’s t-test; Fig. 6).
Fig. 3.
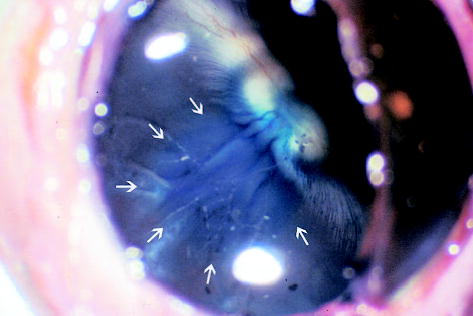
Gross picture of a rabbit eye 4 weeks after subretinal injection of the second-generation feline immunodeficiency virus vector showing the blue-stained round area (arrows) in the retina that was the retinal bleb created by the subretinal injection (original magnification, ×49.5).
Fig. 4.
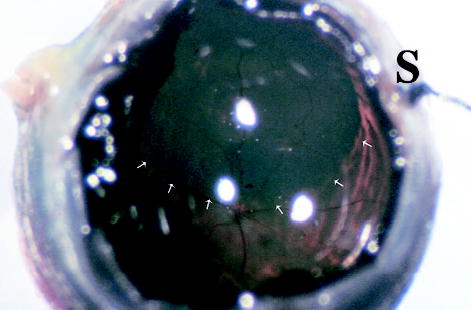
Gross picture of a rat eye 18 months after subretinal injection of the second-generation feline immunodeficiency virus vector showing the retinal bleb area stained blue (arrows). The optic nerve head and major retinal vessels are visible with normal appearance. A 12-o’clock suture (S) can be seen out of focus (original magnification, ×115).
Fig. 5.
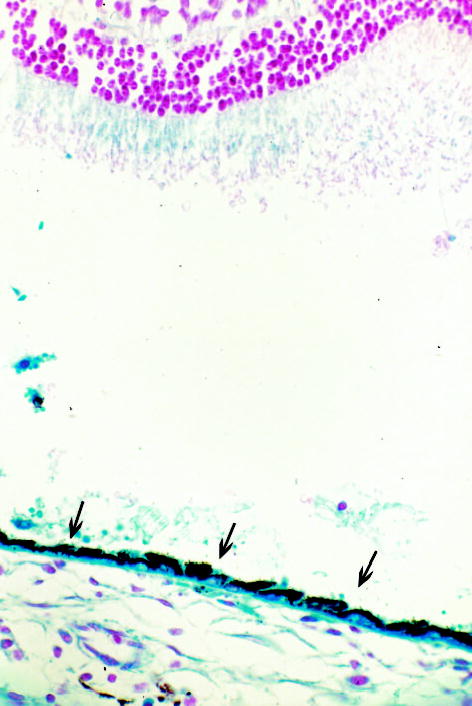
Microscopic photograph of a rabbit eye 2 weeks after subretinal injection of the second-generation feline immunodeficiency virus vector showing continuous retinal pigment epithelium cell blue staining with normal morphology (arrows) and artificially detached normal retina in which some areas of the outer segment were lightly blue stained due to diffusion of the transgene product from the adjacent retinal pigment epithelium (original magnification, ×100; paraffin section, 5 μm; neutral red staining).
Fig. 6.
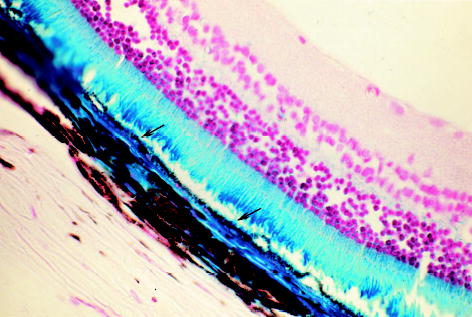
Microscopic photograph of a rat eye 24.25 months after subretinal injection of the second-generation feline immunodeficiency virus vector showing retinal pigment epithelium (RPE) cells stained blue (arrows) with normal morphology. The corresponding areas of the blue-stained retinal outer segment and choroid to the RPE staining area were obviously from diffusion of the transgene product from the RPE (original magnification, ×62.5; paraffin section, 5 μm; neutral red staining).
Pathologic Evaluation of Safety and Toxicity
No toxicity was detected in retinal cells transduced with vector, all of which demonstrated normal morphology. There were more inflammatory cells in the vitreous of vector-injected rabbit eyes than in control eyes from the high-dose first-generation vector group (32 vs. 8, respectively; P = 0.0472, t-test) until 4 weeks after inoculation. More inflammatory cells were also found in vector-injected eyes than in control eyes in the low-dose group (35 vs. 19, respectively), although the difference was not statistically significant (P = 0.28). Similarly, rabbit eyes that received subretinal injections of the second-generation vector pVC-LacZwP had more inflammatory cells in the vitreous than fellow eyes that received the control injection (difference = 65; P = 0.009, paired t-test). In contrast, rat eyes that received subretinal injections of pVC-LacZwP did not have any evidence of toxicity compared with their fellow eyes. Inflammation in the rabbit vitreous of vector-injected eyes peaked around week 3 after the vector injection and then subsided.
Discussion
Recently, FIV vectors have been used for delivering reporter genes or therapeutic genes into the ocular cells of laboratory animals.23–27 These studies have demonstrated the potential of FIV vector–mediated gene transfer into different ocular cell types, according to the method of vector administration employed. The current study provides data on FIV vector transduction (at different dose levels with two vectors) in two commonly used species of experimental animals as well as the longest follow-up yet available of transgene expression and potential toxicity. This work demonstrates that a high-titer second-generation FIV vector can efficiently transfer the LacZ reporter gene into RPE and ganglion cells in both rabbit and rat eyes, as well as into rabbit Müller cells, after subretinal vector administration. Persistent expression without loss of transduced cells, chronic inflammation, or reduction of intensity of expression was observed.
In rabbits, intravitreous or subretinal injection of both FIV vectors was associated with transient vitreous and optic nerve inflammation without other evidence of toxicity. Inflammation peaked 3 weeks after inoculation and subsided without pathologic findings. Although electroretinograms showed a shorter b-wave implicit time in vector-injected eyes, typically an abnormal electroretinogram would show a longer implicit time, indicating the delayed signal transmission. This change could be due to random fluctuation in this small sample size or to direct or indirect (e.g., inflammatory) vector-induced changes in the status of the bipolar cell membrane. This response was not seen in rat eyes; no ocular toxicity was seen during the experiment. The rabbit eye is noted to be very sensitive to foreign substances and may be a better way to assess the inflammatory potential of retinal gene therapy vectors, whereas many vector studies to date have been performed in rodent eyes only.8,10,27,32,33 No inflammation was observed in eyes with control injections in both species. It could be argued that the use of an “empty” vector control would have been more desirable than use of medium or diluent as a control, because this might allow the potential of the particular transgene to elicit inflammatory responses to be assessed. However, it is technically difficult to assure “equivalence” of concentration (titration must be performed using polymerase chain reaction) of such control vectors.
Intravitreal injection in rabbit eyes did not produce any ocular cell transduction. Intravitreal injection has previously been noted to be less efficient than the subretinal route.27,34 This is likely due to the viscosity of the rabbit vitreous and the low concentration of the vector used in these experiments. A dose response was clearly evident between low and high titers of the first-generation FIV vector, with the higher-titer vector leading to higher transduction rates, as expected. The RPE was the dominant cell type transduced by the vector (up to 95% in rabbits and 50% in rats with pVC-LacZwP). In rats, a few ganglion cells located near the injection site were transduced in some eyes. Transduced retinal ganglion cells demonstrated normal morphology. It is possible that vector leaking from the subretinal space at the injection site created a local pocket of vitreous filled with a relatively high concentration of the viral vector, driving transduction of adjacent ganglion cells. It is possible that without the thick cortical vitreous barrier (e.g., after vitrectomy), intravitreal injection of the high-titer second-generation FIV vector could lead to more efficient transduction of ganglion cells and, possibly, other inner retinal cell types. No transgene expression was found in photoreceptor cells or in any anterior segment cell types, unlike what has been reported for other vectors after intravitreal injection.25,26 This difference may result from the use of different species or the age of the animal used,8 differences in vectors or vector preparations used, or blocks to expression via FIV vectors in certain cell types.35 In particular, the use of rat pups,27 known to be more susceptible to transduction,8 rather than adult rats may not be comparable.
This selective targeting or high specificity for RPE cells might be dependent on the pseudotyping envelope used (VSV-G), reflecting the density of receptor on RPE cells, differences in efficiency of vector integration, or differential activity of the transgene promoter (CMV-IE) in other retinal cell types. Selectivity for RPE cells could be a very useful property for strategies to treat certain retinal diseases, such as choroidal neovascularization in age-related macular degeneration, without interfering with other retinal cell functions. For example, pigmented epithelium–derived factor is synthesized in the RPE and secreted into the subretinal space to balance the action of vascular endothelial growth factor, inhibiting choroidal neovascularization.36–41 Delivering the pigmented epithelium–derived factor gene into RPE cells has been shown to inhibit choroidal neovascularization in a laser-induced choroidal neovascularization rat model.42 Stable gene transfer to RPE cells and the persistent transgene expression mediated by the second FIV vector described in the current study could have important clinical applications. Second- or third-generation FIV vectors merit further testing for safety and efficiency of therapeutic gene transfer in both small animal and eventually in nonhuman primate models.
Footnotes
Supported by the National Institutes of Health (EY07366 to W.R.F. and AI45992 to D.J.L.) and by the UCSD Center for AIDS Research (NIH P30 AI36214) and a gift from Vision of Children Foundation, San Diego, CA.
References
- 1.Bennett J, Maguire AM, Cideciyan AV, et al. Stable transgene expression in rod photoreceptors after recombinant adeno-associated virus-mediated gene transfer to monkey retina. Proc Natl Acad Sci USA. 1999;96:9920–9925. doi: 10.1073/pnas.96.17.9920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Borras T, Tamm ER, Zigler JS., Jr Ocular adenovirus gene transfer varies in efficiency and inflammatory response. Invest Ophthalmol Vis Sci. 1996;37:1282–1293. [PubMed] [Google Scholar]
- 3.Cayouette M, Gravel C. Adenovirus-mediated gene transfer of ciliary neurotrophic factor can prevent photoreceptor degeneration in the retinal degeneration (rd) mouse. Hum Gene Ther. 1997;8:423–430. doi: 10.1089/hum.1997.8.4-423. [DOI] [PubMed] [Google Scholar]
- 4.Dudus L, Anand V, Acland GM, et al. Persistent transgene product in retina, optic nerve and brain after intraocular injection of rAAV. Vision Res. 1999;39:2545–2553. doi: 10.1016/s0042-6989(98)00308-3. [DOI] [PubMed] [Google Scholar]
- 5.Flannery JG, Zolotukhin S, Vaquero MI, LaVail MM, Muzyczka N, Hauswirth WW. Efficient photoreceptor-targeted gene expression in vivo by recombinant adeno-associated virus. Proc Natl Acad Sci USA. 1997;94:6916–6921. doi: 10.1073/pnas.94.13.6916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Galileo DS, Hunter K, Smith SB. Stable and efficient gene transfer into the mutant retinal pigment epithelial cells of the Mitf(vit) mouse using a lentiviral vector. Curr Eye Res. 1999;18:135–142. doi: 10.1076/ceyr.18.2.135.5376. [DOI] [PubMed] [Google Scholar]
- 7.Grant CA, Ponnazhagan S, Wang XS, Srivastava A, Li T. Evaluation of recombinant adeno-associated virus as a gene transfer vector for the retina. Curr Eye Res. 1997;16:949–956. doi: 10.1076/ceyr.16.9.949.5046. [DOI] [PubMed] [Google Scholar]
- 8.Miyoshi H, Takahashi M, Gage FH, Verma IM. Stable and efficient gene transfer into the retina using an HIV-based lentiviral vector. Proc Natl Acad Sci USA. 1997;94:10319–10323. doi: 10.1073/pnas.94.19.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rolling F, Shen WY, Tabarias H, et al. Evaluation of adeno-associated virus-mediated gene transfer into the rat retina by clinical fluorescence photography. Hum Gene Ther. 1999;10:641–648. doi: 10.1089/10430349950018715. [DOI] [PubMed] [Google Scholar]
- 10.Spencer B, Agarwala S, Miskulin M, Smith M, Brandt CR. Herpes simplex virus-mediated gene delivery to the rodent visual system. Invest Ophthalmol Vis Sci. 2000;41:1392–1401. [PubMed] [Google Scholar]
- 11.Akimoto M, Miyatake S, Kogishi J, et al. Adenovirally expressed basic fibroblast growth factor rescues photoreceptor cells in RCS rats. Invest Ophthalmol Vis Sci. 1999;40:273–279. [PubMed] [Google Scholar]
- 12.Cayouette M, Behn D, Sendtner M, Lachapelle P, Gravel C. Intraocular gene transfer of ciliary neurotrophic factor prevents death and increases responsiveness of rod photoreceptors in the retinal degeneration slow mouse. J Neurosci. 1998;18:9282–9293. doi: 10.1523/JNEUROSCI.18-22-09282.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kafri T, Blomer U, Peterson DA, Gage FH, Verma IM. Sustained expression of genes delivered directly into liver and muscle by lentiviral vectors. Nat Genet. 1997;17:314–317. doi: 10.1038/ng1197-314. [DOI] [PubMed] [Google Scholar]
- 14.Naldini L, Blomer U, Gallay P, et al. In vivo gene delivery and stable transduction of nondividing cells by a lentiviral vector. Science. 1996;272:263–267. doi: 10.1126/science.272.5259.263. [DOI] [PubMed] [Google Scholar]
- 15.Lewis PF, Emerman M. Passage through mitosis is required for oncoretroviruses but not for the human immunodeficiency virus. J Virol. 1994;68:510–516. doi: 10.1128/jvi.68.1.510-516.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nomura T, Yabe T, Mochizuki H, Reiser J, Becerra SP, Schwartz JP. Survival effects of pigment epithelium-derived factor expressed by a lentiviral vector in rat cerebellar granule cells. Dev Neurosci. 2001;23:145–152. doi: 10.1159/000048706. [DOI] [PubMed] [Google Scholar]
- 17.Cheng L, Chaidhawangul S, Wong-Staal F, et al. Human immunodeficiency virus type 2 (HIV-2) vector-mediated in vivo gene transfer into adult rabbit retina. Curr Eye Res. 2002;24:196–201. doi: 10.1076/ceyr.24.3.196.8302. [DOI] [PubMed] [Google Scholar]
- 18.Emerman M. From curse to cure: HIV for gene therapy? Nat Biotechnol. 1996;14:943. doi: 10.1038/nbt0896-943. [DOI] [PubMed] [Google Scholar]
- 19.Olmsted RA, Hirsch VM, Purcell RH, Johnson PR. Nucleotide sequence analysis of feline immunodeficiency virus: genome organization and relationship to other lentiviruses. Proc Natl Acad Sci USA. 1989;86:8088–8092. doi: 10.1073/pnas.86.20.8088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hartmann K. Feline immunodeficiency virus infection: an overview. Vet J. 1998;155:123–137. doi: 10.1016/S1090-0233(98)80008-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Curran MA, Kaiser SM, Achacoso PL, Nolan GP. Efficient transduction of nondividing cells by optimized feline immunodeficiency virus vectors. Mol Ther. 2000;1:31–38. doi: 10.1006/mthe.1999.0007. [DOI] [PubMed] [Google Scholar]
- 22.Poeschla EM, Wong-Staal F, Looney DJ. Efficient transduction of nondividing human cells by feline immunodeficiency virus lentiviral vectors. Nat Med. 1998;4:354–357. doi: 10.1038/nm0398-354. [DOI] [PubMed] [Google Scholar]
- 23.Loewen N, Fautsch MP, Peretz M, et al. Genetic modification of human trabecular meshwork with lentiviral vectors. Hum Gene Ther. 2001;12:2109–2119. doi: 10.1089/10430340152677449. [DOI] [PubMed] [Google Scholar]
- 24.Loewen N, Bahler C, Teo WL, et al. Preservation of aqueous outflow facility after second-generation FIV vector-mediated expression of marker genes in anterior segments of human eyes. Invest Ophthalmol Vis Sci. 2002;43:3686–3690. [PubMed] [Google Scholar]
- 25.Derksen TA, Sauter SL, Davidson BL. Feline immunodeficiency virus vectors. Gene transfer to mouse retina following intravitreal injection. J Gene Med. 2002;4:463–469. doi: 10.1002/jgm.267. [DOI] [PubMed] [Google Scholar]
- 26.Lotery AJ, Derksen TA, Russell SR, et al. Gene transfer to the nonhuman primate retina with recombinant feline immunodeficiency virus vectors. Hum Gene Ther. 2002;13:689–696. doi: 10.1089/104303402317322258. [DOI] [PubMed] [Google Scholar]
- 27.Loewen N, Leske DA, Chen Y, et al. Comparison of wild-type and class I integrase mutant-FIV vectors in retina demonstrates sustained expression of integrated transgenes in retinal pigment epithelium. J Gene Med. 2003;5:1009–1017. doi: 10.1002/jgm.447. [DOI] [PubMed] [Google Scholar]
- 28.Morris KV, Gilbert J, Wong-Staal F, Gasmi M, Looney D. Transduction of cell lines and primary cells by FIV packaged HIV vectors. Mol Ther. 2004;10:181–190. doi: 10.1016/j.ymthe.2004.03.019. [DOI] [PubMed] [Google Scholar]
- 29.Cheng L, Hostetler KY, Gardner MF, et al. Intravitreal toxicology in rabbits of two preparations of 1-O-octadecyl-sn-glycerol-3-phosphonoformate, a sustained-delivery anti-CMV drug. Invest Ophthalmol Vis Sci. 1999;40:1487–1495. [PubMed] [Google Scholar]
- 30.Cheng L, Hostetler KY, Chaidhawangul S, et al. Treatment or prevention of herpes simplex virus retinitis with intravitreally injectable crystalline 1-O-hexadecylpropanediol-3-phospho-ganciclovir. Invest Ophthalmol Vis Sci. 2002;43:515–521. [PubMed] [Google Scholar]
- 31.Mohamed HE, Cheng L, Bartsch DK, et al. Preventive versus treatment effect of Ag3340, a potent matrix metalloproteinase inhibitor in a rat model of choroidal neovascularization. J Ocul Pharmacol Ther. 2004;20:217–236. doi: 10.1089/1080768041223657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ali RR, Reichel MB, De Alwis M, et al. Adeno-associated virus gene transfer to mouse retina. Hum Gene Ther. 1998;9:81–86. doi: 10.1089/hum.1998.9.1-81. [DOI] [PubMed] [Google Scholar]
- 33.Bennett J, Wilson J, Sun D, Forbes B, Maguire A. Adenovirus vector-mediated in vivo gene transfer into adult murine retina. Invest Ophthalmol Vis Sci. 1994;35:2535–2542. [PubMed] [Google Scholar]
- 34.Pitkanen L, Ruponen M, Nieminen J, Urtti A. Vitreous is a barrier in nonviral gene transfer by cationic lipids and polymers. Pharm Res. 2003;20:576–583. doi: 10.1023/a:1023238530504. [DOI] [PubMed] [Google Scholar]
- 35.Price MA, Case SS, Carbonaro DA, et al. Expression from second-generation feline immunodeficiency virus vectors is impaired in human hematopoietic cells. Mol Ther. 2002;6:645–652. [PubMed] [Google Scholar]
- 36.Gao G, Li Y, Gee S, et al. Down-regulation of vascular endothelial growth factor and up-regulation of pigment epithelium-derived factor: a possible mechanism for the anti-angiogenic activity of plasminogen kringle 5. J Biol Chem. 2002;277:9492–9497. doi: 10.1074/jbc.M108004200. [DOI] [PubMed] [Google Scholar]
- 37.Gao G, Li Y, Zhang D, Gee S, Crosson C, Ma J. Unbalanced expression of VEGF and PEDF in ischemia-induced retinal neovascularization. FEBS Lett. 2001;489:270–276. doi: 10.1016/s0014-5793(01)02110-x. [DOI] [PubMed] [Google Scholar]
- 38.Holekamp NM, Bouck N, Volpert O. Pigment epithelium-derived factor is deficient in the vitreous of patients with choroidal neovascularization due to age-related macular degeneration. Am J Ophthalmol. 2002;134:220–227. doi: 10.1016/s0002-9394(02)01549-0. [DOI] [PubMed] [Google Scholar]
- 39.King GL, Suzuma K. Pigment-epithelium-derived factor—a key coordinator of retinal neuronal and vascular functions. N Engl J Med. 2000;342:349–351. doi: 10.1056/NEJM200002033420511. [DOI] [PubMed] [Google Scholar]
- 40.Mori K, Gehlbach P, Yamamoto S, et al. AAV-mediated gene transfer of pigment epithelium-derived factor inhibits choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1994–2000. [PubMed] [Google Scholar]
- 41.Renno RZ, Youssri AI, Michaud N, Gragoudas ES, Miller JW. Expression of pigment epithelium-derived factor in experimental choroidal neovascularization. Invest Ophthalmol Vis Sci. 2002;43:1574–1580. [PubMed] [Google Scholar]
- 42.Mori K, Duh E, Gehlbach P, et al. Pigment epithelium-derived factor inhibits retinal and choroidal neovascularization. J Cell Physiol. 2001;188:253–263. doi: 10.1002/jcp.1114. [DOI] [PubMed] [Google Scholar]


