Abstract
Objective
The purpose of this study was to determine whether pre–B-cell colony–enhancing factor is a secreted cytokine in the human amnion and to study its chemotaxic and antiapoptotic properties.
Study design
Pre–B-cell colony–enhancing factor secretion was studied from amniotic epithelial-like WISH cells and primary amniotic epithelial cells that were seeded on squares of immobilon-P membrane and stimulated with lipopolysaccharide or tumor necrosis factor-α , respectively. The pre–B-cell colony–enhancing factor protein was detected both intracellularly and after secretion, as bound to the membrane, by immunostaining and densitometry. Medium and cell lysates that were obtained from WISH cells that were treated with lipopolysaccharide alone or together with a pre–B-cell colony–enhancing factor antisense oligonucleotide to block pre–B-cell colony–enhancing factor translation were also analyzed for secreted pre–B-cell colony–enhancing factor by Western blotting and densitometry. A chemotaxic effect of pre–B-cell colony–enhancing factor on human neutrophils was compared with the chemoattractants interleukin-8 and N-Formyl-Met-Leu-Phe methyl ester in a rapid fluorescence-based neutrophil migration assay. Apoptosis was induced in primary amniotic epithelial cells and fibroblasts by actinomycin D (1 μg/mL); the antiapoptotic effects of pre–B-cell colony–enhancing factor on early apoptosis were measured by the annexin V assay, and the late effects were determined by measurement of nuclear matrix protein in the media.
Results
Treatment of amnion cells that adhered to immobilon-P membrane to induce the secretion of pre–B-cell colony–enhancing factor showed significantly (P < .05) more pre–B-cell colony–enhancing factor protein surrounding the cells compared with the controls. Although the addition of lipopolysaccharide to cultured WISH cells caused the secretion of pre–B-cell colony–enhancing factor into the medium, co-treatment with an antisense oligonucleotide to pre–B-cell colony–enhancing factor obliterated it. Analysis of the cell lysates showed no significant change, which suggests that most of the pre–B-cell colony–enhancing factor protein had been secreted. No significant chemotaxic effects of pre–B-cell colony–enhancing factor were observed; however, pre–B-cell colony–enhancing factor treatment (100 ng/mL), together with actinomycin D, cancelled the early induction of apoptosis, although there was a dose-dependent and significant late antiapoptotic effect on primary amnion epithelial cells (P < .001) and fibroblasts (P < .01).
Conclusion
Pre–B-cell colony–enhancing factor is a secreted protein from amniotic epithelial cells. Although it had no chemotaxic effects, it was antiapoptotic for both amniotic epithelial cells and fibroblasts and may protect these cells against apoptosis that is induced by chronic distension, labor, or infection.
Keywords: Pre-B-cell colony-enhancing factor, Cytokine, Secretion, Chemotaxis, Apoptosis
Pre–B-cell colony–enhancing factor (PBEF) was first identified from activated peripheral human blood lymphocytes and shown to act as a growth factor for B cells, which suggests its involvement in the immune cell response.1 More recently, it has been identified as an enzyme, nicotinamide phosphoribosyltransferase, that is involved in the biosynthesis of nicotinamide adenine dinucleotide (NAD).2 NAD is an essential coenzyme for the regulation of many metabolic pathways by its transfer of hydrogen or electrons during cellular respiration. In addition, NAD also functions as a precursor of molecules that are involved in other major regulatory metabolic pathways.2 However, these actions are all intracellular, in contrast to the extracellular cytokine-like activities that we have suggested for PBEF from our studies with the human fetal membranes.3–5 In these studies, we have shown the up-regulation of the PBEF gene by the in vitro distension of fetal membrane explants,3 as well as in vivo, by both normal term and preterm labor.5 However, the PBEF gene was also up-regulated by both severe intrauterine infection (chorioamnionitis) and the in vitro addition of lipopolysaccharide.4 These observations suggested that PBEF might link the sterile stretch-induced pathway and the infection-mediated pathway to labor.6 Thus, we studied the acute gene effects that are caused by the addition of PBEF to term fetal membrane explants and showed it to induce changes in some chemokines and inflammatory cytokines, as well as prostaglandin-endoperoxide synthase 2,6 which are all important in the induction of labor.
Because PBEF has no signal sequence in its amino acid structure for its secretion and has no homology with any other cytokines, it has been suggested that PBEF cannot be a secreted cytokine-like protein.7 However, it has been shown recently that PBEF is secreted by neutrophils and has antiapoptotic properties for these cells.8 Thus, the aim of our study was to extend this to show that PBEF is indeed secreted from the human amniotic epithelium and that it could be stimulated by such inflammatory agents as lipopolysac-charide and tumor necrosis factor (TNF) and specifically blocked in vitro. Although nothing is known about its receptor or mechanism of action, we have tried to further define some of its actions as a cytokine in this tissue. We therefore studied its chemotaxic properties for human neutrophils or polymorphonuclear cells and whether it affects the induced apoptosis of human amnion cells, actions that would further justify its classification as a cytokine and better define its autocrine/paracrine actions in the fetal membranes.
Material and methods
Patients and tissues
Fetal membranes with adhering decidua were collected from patients at elective cesarean delivery at term (38–40 weeks of gestation), before labor, and within 30 minutes of expulsion (n = 11). Institutional review board approval was obtained, but consent was not required because these tissues were collected anonymously. None of the patients had clinical signs of infection; the placentas and membranes were examined histologically by 1 pathologist to eliminate localized infection, as previously reported.5
Isolation of primary amnion cells
Primary amniotic epithelial cells and fibroblasts were isolated by the procedure of Casey and MacDonald.9 In brief, the amnion layer was stripped from the adjacent choriodecidua; the epithelial cells were isolated by 2 trypsin (0.2%) digestions, and the fibroblasts were isolated after the removal of epithelial cells by the additional incubation with 0.75 mg/mL collagenase A. The purity that was obtained with each isolation was similar to that previously reported.9 Isolated primary epithelial cells showed only 2% fibroblast contamination, although isolated fibroblasts showed 10% contamination with primary amniotic epithelial cells. The cells were used without passages.
Demonstration of the secretion of PBEF
To show the secretion of the PBEF protein, an amniotic epithelial-like cell line (WISH) was obtained from American Type Culture Collection (No.CCL-25; ATCC, Manassas, Va), and primary amniotic epithelial cells were seeded onto squares of immobilon-P membrane filter (Millipore, Bedford, Mass), placed on the bottom of 24-well plates in OptiMEM-I medium (Invitrogen, Carlsbad, Calif) without antibiotics or fetal calf serum (FCS). The cells attach to this substrate, and their secreted products become firmly bound to the membrane. The cells (10,000) were seeded in each well, incubated overnight at 37° C in 95% oxygen/5% carbon dioxide, and treated with either 50 ng/mL lipopolysaccharide (WISH cells) or TNFα (30 ng/mL; primary cells) for 4 hours. After this, to visualize the PBEF protein by immunostaining, the cells and their products were fixed on the membrane by treatment with Bouin’s fixative for 5 minutes and washed with phosphate-buffered saline (PBS) 3 times for 3 minutes each; the nonspecific binding sites were blocked by treatment in bovine serum albumin (BSA) in PBS for 1 hour. Washes were repeated with PBS that contained 0.1% BSA, followed by incubation with the immunoglobulin G (IgG) fraction derived from a rabbit polyclonal antibody to human PBEF1 (1/1000 dilution; supplied by Dr C. Saris, Amgen Inc, Thousand Oaks, Calif) for 2 hours, then washed again, and incubated with 1/2000 dilution of biotinylated anti-rabbit IgG (Vector Laboratories, Inc, Burlingame, Calif) for 1 hour. For the negative control, the IgG fraction that was derived from normal rabbit serum was used at the same concentration as the primary antibody. After 3 further washes, avidinbiotin-peroxidase complex reagent from an Elite Kit (Vector Laboratories Inc) was added for 45 minutes and washed again. The color was developed with diaminobenzidine (0.5 mg/mL) added for 3 minutes followed by several water rinses. The blots were allowed to air-dry before they were mounted on microscope slides. The results are from 3 independent experiments with both WISH and primary cells from 3 different patients.
Imaging and analysis
Microscopic images were obtained with a photomicroscope (Olympus BX 51; Olympus America Inc, Melville, NY). The average staining intensity and total immunostained area (cell plus its secreted PBEF) were performed for each cell (30 cells per slide, 3 slides for each experimental group) with Olympus software (Microsuite; Olympus America Inc). The results are shown as means and SDs that were calculated for each experimental group. The nonparametric Mann-Whitney test was used to calculate the statistical significance of the difference that was observed between 2 groups.
Western analysis of secreted PBEF
WISH cells (300,000 per well) were seeded into 6-well plates in DMEM-F12 growth medium that was supplemented with 10% FCS. After reaching 80% confluency, the growth medium was replaced with DMEM-F12 (2 mL/well) and 0.5% FCS. Lipopolysaccharide (50 ng/mL) was added alone to induce PBEF secretion or together with an antisense oligonucleotide (10 μmol/L phosphorothioated oligonucleotide) to prevent the translation of the PBEF protein.8 The addition of a sense oligonucleotide, together with lipopolysaccharide was used to assess any nonspecific effects of these oligonucleotides. These sequences were antisense oligonucleotide, 5′-T*T*C*T-GCCGCAGGATTCATCTC*G*G*G-3′ and sense oligonucleotide, 5′ -C*C*C*GAGATGAATCCTGCG-GCA*G*A*A-3′ . [Asterisks mark the positions of the addition of phosphorothioate]. Controls were incubated in unconditioned media only. After 72 hours, the media were collected and concentrated to approximately 50 μL per sample using Centricon 30 filtration units (Millipore, Bedford, Mass). The protein concentration of each sample was measured with the BioRad Protein Assay (Biorad, Hercules, Calif). Known concentrations of BSA were used for a standard curve. Total protein (20 μg) per sample was loaded on a 10% sodium dodecylsulfate polyacrylamide gel electrophoresis and transferred to a Hybond-P polyvinylidene fluoride membrane (Amersham Biosciences, Piscataway, NJ). Western blotting was performed by blocking polyvinylidene fluoride membranes in PBS that contained 0.1% Tween 20 and 3% BSA for 1 hour, followed by washes with PBS that contained 0.1%Tween-20 and incubation with a primary PBEF antibody (Amgen Inc), diluted 1/2000 in the blocking buffer. After being washed, the blots were incubated with the biotinylated secondary antibody (1/2000 dilution) for 1 hour, washed, and incubated with avidinbiotin-peroxidase complex reagent (Vector Laboratories Inc) for 45 minutes, then washed again, and developed with the enhanced chemiluminescence kit (Amersham Inc). The blots were immediately exposed to hyperfilm-enhanced chemiluminescence, and the signals were quantitated with a densitometer (Kodak EDAS290 System; Eastman Kodak Co, Rochester, NY).
The WISH cells from these experiments were collected after trypsinization to lift them from the wells and were counted. Cells (2 × 106) from each well were lysed by the addition of 50 μL of lysis buffer (R&D Systems, Minneapolis, Minn) followed by a 15-minute incubation in ice. The samples were centrifuged at 13,000 rpm for 1 minute, and the supernatants were transferred to new microcentrifuge tubes. The protein contents of each sample were measured (as described earlier), and 20 μg from each sample was loaded on sodium dodecylsulfate polyacrylamide gel electrophoresis and transferred to a Hybond-P polyvinylidene fluoride membrane. Signal detection was performed as described earlier. All experiments were repeated at least 3 times, and the results that are shown represent the average of these experiments.
The chemotaxic effect of PBEF on human neutrophils
Heparinized whole blood was collected from healthy donors in accordance with an approved institutional review board protocol. Neutrophils from 3 donors were isolated by the method of Boyum.10 Briefly, the anticoagulated blood was layered onto a density gradient (Histopaque-1077; Sigma Diagnostics Inc, St. Louis, Mo) and centrifuged at 400g for 30 minutes to separate the neutrophils from the peripheral blood mononuclear cells. The supernatant that included the peripheral blood mononuclear cell layer was aspirated and discarded. The sides of the tube were swabbed to remove any residual cells. The remaining red blood cell pellet was resuspended in a small volume of PBS solution and lysed with a hypotonic solution. The resulting neutrophil pellet was washed with PBS and resuspended in RPMI-1640 (Sigma Diagnostics Inc) that contained 10% heat-treated FCS. Calcein AM (5 μg/mL; Molecular Probes, Eugene, Ore) was added to the suspension of cells in RPMI-FCS and incubated at 37° C for 30 minutes.11 The neutrophils were washed twice with PBS and resuspended in RPMI-FCS to a concentration of 2 × 106cells/mL. The standard chemotactic factors, interleukin-8 (Sigma Diagnostics Inc), and N-Formyl-Met-Leu-Phe methyl ester (fMLP; Sigma Diagnostics Inc) were diluted in PBS with 0.1% human serum albumin to selected concentrations (10−7 to 10−9 mol/L and 10−6 to 10−8 mol/L, respectively). Recombinant human PBEF that was produced as previously described5 was also diluted in the PBS-human serum albumin buffer to concentrations of 2 × 10−7 mol/L to 2 × 10−9 mol/L. A reusable chemotaxis chamber (Neuroprobe, Gaithersburg, Md) with a disposable 96-well low-volume plate was used to determine neutrophil migration with a well-established method.12,13 The diluted interleukin-8 (IL-8), fMLP, PBEF, or the negative control (PBS-human serum albumin) were loaded into the bottom wells of the 96-well plate. To determine the total fluorescence of the neutrophils, 25 μL of calcein-labeled cell suspensions were also loaded into at least of 3 bottom wells per 96-well plate. The same volume of cells was loaded on top of the polyvinylpytrolidone–free polycarbonate filter that was positioned on top of the plate in the chamber. The chamber was incubated at 37° C, 5% carbon dioxide for 1 hour. The plate with the attached filter was removed from the chamber, and the non-migrating cells that remained on the top of the filter were removed by gentle aspiration and/or wiping with a tissue. The plate was read on a fluorescent plate reader (Victor II; Perkin Elmer Life Sciences Inc, Boston, Mass). Migration into the bottom well was measured by the calcein fluorescence signal (excitation, 485 nm; emission, 530 nm). The results are shown as an average of 11 independent experiments; significance was determined by 1-way analysis of variance.
Apoptosis
The effects of PBEF on early- and late-stage apoptosis were determined on primary amniotic epithelial cells and fibroblasts. To study the effects of PBEF on early apoptosis, cells were seeded on Nunc chamber slides and grown to 80% confluency; apoptosis was induced by the addition of actinomycin D (1 μg/mL) alone or in the presence of PBEF(10 or 100 ng/mL), and controls were incubated in media alone. After 4 hours, the cells were washed with cold PBS, and the Vybrant Apoptosis Assay (Molecular Probes) was used to detect apoptotic and necrotic cells. Early in the apoptotic process, cell surface phospholipid asymmetry is disrupted, which leads to the exposure of phosphatidylserine on the outer leaflet of the cytoplasmic membrane. The Vybrant Apoptosis Assay is based on recognition and the binding of annexin V to the phosphatidylserine on the cell surface, which is an early indicator of apoptosis. Annexin V is conjugated to fluorescent dye Alexa Fluor 488, which causes the apoptotic cells to show green fluorescence, although the propidium iodide (another component of this assay) is a nucleic acid–binding dye that is impermeable to live and apoptotic cells but stains necrotic cells with red fluorescence. After the incubation with annexin V and propidium iodide for 15 minutes, the cells were washed with annexin-binding buffer (supplied by the manufacturer) and mounted. The apoptotic and necrotic cells were visualized with fluorescent microscopy, although live cells remained invisible. The fluorescent cells were counted in 30 fields per slide. These experiments were repeated 3 times.
To study the effects of PBEF on late apoptosis, the cells were seeded in 6-well plates at a density of 300,000 cells per well and treated with actinomycin D (1μg/mL) alone or together with PBEF (1, 10, or 100 ng/mL), in OptimMEM-I medium without FCS or antibiotics. Controls were incubated in medium alone. After 72 hours, the media were collected, and apoptosis was measured with the nuclear matrix protein enzyme-linked immunosorbent assay (Calbiochem, Temecula, Calif), according to the manufacturer’s protocol. This protein is released only into the medium by apoptotic cells, and the extent of its release correlates directly with the amount of apoptosis. Although some apoptosis is usually present in the controls, this rises dramatically when apoptosis is induced (such as with actinomycin D). These experiments were repeated 4 times with primary cells that were derived from different patients. Significance was determined by the Kruskal-Wallis test.
Results
The secretion of PBEF
After 4 hours of treatment with lipopolysaccharide, WISH cells secreted their PBEF protein, which remained immobilized on the immobilon membrane and is shown after immunostaining as a halo surrounding each secreting cell (Figure 1, A), this was absent in the nontreated cells (Figure 1, B). Similarly, the TNFα treatment caused the primary amniotic epithelial cells to secrete PBEF, which is seen after immunostaining (Figure 1, C). However, in these cells, the PBEF secretion was more diffuse and did not show such a clean halo. The nontreated cells showed no evidence of secreted PBEF (Figure 1, D). The secretion of PBEF from the WISH cells was confirmed in primary amniotic epithelial cells and showed that both lipopolysaccharide and TNFα caused its secretion, as described previously for PBEF gene expression.5 The PBEF that was secreted by the primary amniotic epithelial cells was quantitated by densitometry and showed that significantly more PBEF secreted (P < .05) after the TNFα treatment, although the area of secretion by individual cells was quite variable, which contributed to a large standard deviation (Figure 2).
Figure 1.
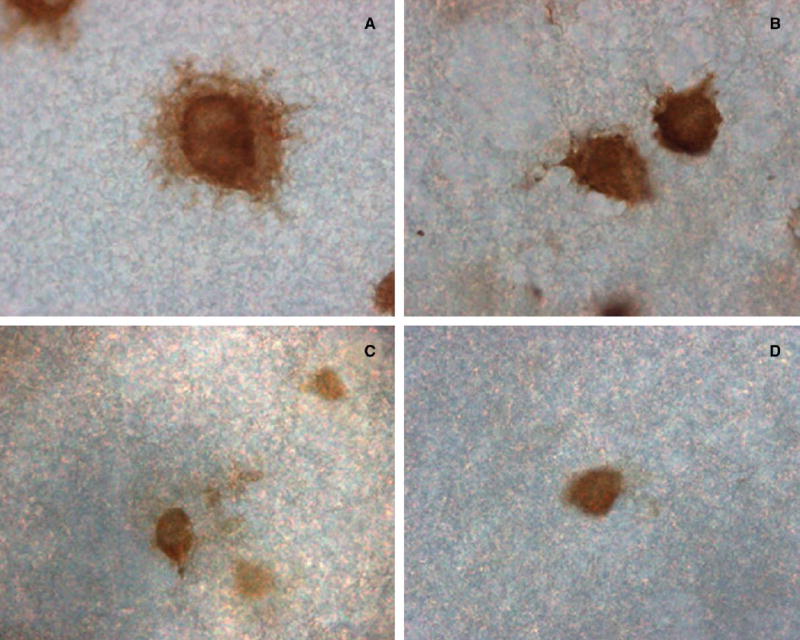
PBEF protein secretion from a representative amnion-derived WISH cell that was grown on immobilon-P membrane is shown after a 4-hour treatment with lipopolysaccharide (50 ng/mL) and immunostained to show the intracellular and secreted (extracellular) PBEF (A); there was no such halo of secreted PBEF in the control untreated cell (B). A representative primary amniotic epithelial cell was treated for 4 hours with TNF-α (30 ng/mL); the secreted PBEF was more diffuse but shown as immunostained extracellular patches (C); the extracellular staining was absent in the control (D). (Original magnification, ×400.)
Figure 2.
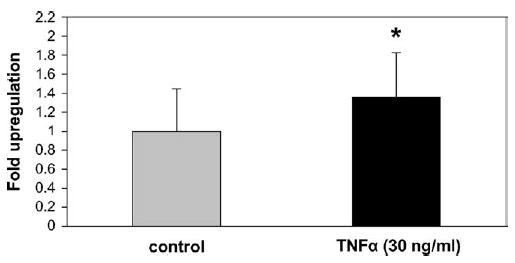
Densitometric analysis (MicroSuite software; Olympus America Inc) is shown as mean ± SD of the PBEF protein that was bound to immobilon-P membranes that were secreted from primary amniotic epithelial cells (n = 90) that were measured as the average intensity and total surface area covered by each cell and the surrounding secreted protein and showed a significant increase after 4 hours of TNF-α (30 ng/mL) treatment compared to the untreated controls by the Mann-Whitney test. The asterisk denotes P < .05.
Western analysis of PBEF secretion and its antisense block
The treatment of WISH cells with lipopolysaccharide caused < 3-fold increase (P < .001) in the amount of PBEF protein secreted into the media, when compared to the control (Figure 3, A). The addition of a sense oligonucleotide together with lipopolysaccharide had little effect on the response to lipopolysaccharide. However, when the antisense oligonucleotide was added with lipopolysaccharide, PBEF secretion was suppressed down to the control levels (P < .05; Figure 3, A). When lysates from these cells were analyzed for PBEF protein retention in the cells, the lipopolysaccharide treatment only marginally increased this, which suggests that lipopolysaccharide caused most of the PBEF to be secreted (Figure 3, B). The addition of either the sense or antisense oligonucleotide together with lipopolysaccharide had little effect on the concentration of PBEF within the cells (Figure 3, B).
Figure 3.
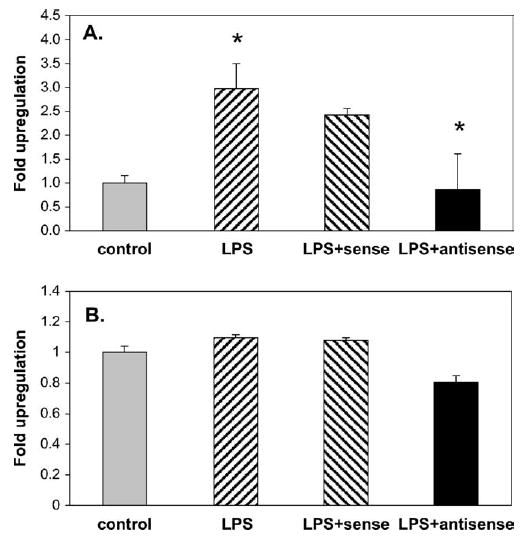
Quantitated Western analysis of PBEF that was secreted by amnion-like WISH cells into the culture media after treatment for 72 hours with lipopolysaccharide (50 ng/mL) is shown as mean ± SD (A) and in cell lysates (B). Lipopolysaccharide treatment significantly (*) increased the PBEF in the medium (P < .001); the addition of a control sense oligonucleotide to the lipopolysaccharide had little effect on this secretion, whereas the addition of the PBEF antisense oligonucleotide together with lipopolysaccharide significantly (*) reduced the secretion of PBEF (P < .05), determined by the Kruskal-Wallis test. Analysis of the PBEF in the cell lysates showed no significant change in the amount of PBEF after the same treatments, which suggests that lipopolysaccharide caused the rapid secretion of the PBEF protein that was induced by the lipopolysaccharide treatment.
The effect of PBEF on neutrophil chemotaxis
The 2 standard chemoattractants, IL-8 and fMLP, were used in this assay as positive controls, and both caused a dose-dependent increase in neutrophil chemotaxis. IL-8 was extremely (P < .001) chemotaxic at 10−7 mol/L and still significant (P < .01) at lower concentrations. The highest concentration of IL-8 caused the migration of 80% of the neutrophils, although the random migration (control) showed only 20% migration (Figure 4). Similarly, fMLP caused significant chemotaxis at all 3 concentrations (10−6, 10−7, and 10−8 mol/L; P < .01, < .05, and < .05, respectively), and it caused the migration of 65% of the neutrophils at its highest concentration. The PBEF, however, caused no neutrophil migration. The experiments were repeated 3 times with blood from 3 different donors with similar results. Because of the high degree of interpatient variability, the results are expressed as means and standard errors for all experiments. These data show that PBEF has no chemoattractant activity compared with IL-8.
Figure 4.
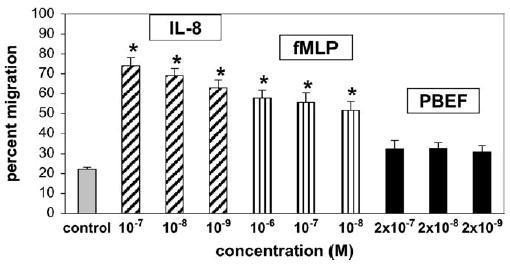
The chemotaxis of human neutrophils, which is shown as the percent of the neutrophils migrating towards a chemoattractant in a standard assay and shown as means ± SD. Significance was determined by 1-way analysis of variance and shown by asterisks. The standards, interleukin-8 and fMLP, were used as positive controls, and both showed significant dose-dependent migration of neutrophils: IL-8 was extremely significant (P < .001) at 10−7 mol/L and still significant at 10−8 and 10−9 mol/L (P < .01); fMLP (10−6 to 10−8 mol/L) also caused significant migration (P < .01, P < .05, and P < .05, respectively). PBEF at considerably higher doses (2 × 10−7 to 2 × 10−9 mol/L) caused no chemotaxis.
The effect of PBEF on apoptosis
The early antiapoptotic effect of PBEF on primary amniotic epithelial cells and fibroblasts at 4 hours was studied with the annexin V assay. The results with the fibroblasts were the most pronounced (Figure 5); the results with the epithelial cells were similar, but the staining intensity for epithelial cells was lower than for fibroblasts, which makes it more difficult to capture a sharp image. The control (Figure 5, A) shows only a few apoptotic cells; the actinomycin D treatment induced apoptosis (Figure 5, B). The addition of PBEF (100 ng/mL) together with the actinomycin D cancelled the apoptosis induction (Figure 5, C). The addition of PBEF alone had no effect and is not shown. Because of the short time of treatment (4 hours), no necrotic cells were observed in this study, and the live cells remained unstained and unseen.
Figure 5.
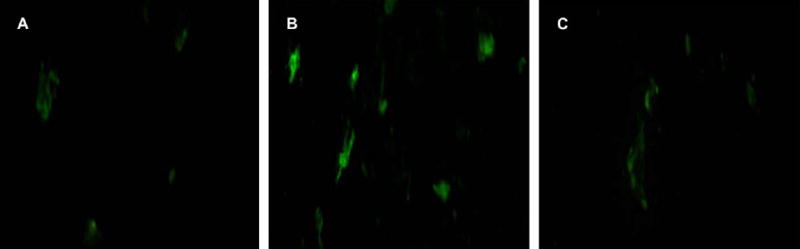
The effect of PBEF on early apoptosis by annexin V staining (green). A, Primary amniotic fibroblasts with no treatment; B, control after the induction of apoptosis by a 4-hour treatment with actinomycin D (1 μg/mL), which increased the numbers of apoptotic cells. C, Cells that were treated with both actinomycin D and PBEF (100 ng/mL) show the reduction in the number of apoptotic cells by the PBEF treatment. Note that the annexin V component of this assay stained apoptotic cells with green fluorescence and that the live cells that are present were unseen and remained dark.
The effects of PBEF on late-stage apoptosis (>72 hours) in primary amniotic epithelial cells were studied by the induction of apoptosis with actinomycin D (1 μg/mL), which caused a significant (P < .001) increase in the number of apoptotic cells, which was measured by the leakage of nuclear matrix protein into the culture medium compared with nontreated controls (Figure 6, A). However, when these cells were treated with both actinomycin D and PBEF (1, 10, or 100 ng/mL), a dose-dependent reduction in the number of apoptotic cells was seen (Figure 6, A). This inhibitory effect of PBEF on actinomycin D–induced apoptosis reached statistical significance (P < .001) with the highest PBEF dose (100 ng/mL) and with an apoptosis level similar to that of the control. PBEF alone had no effect on apoptosis at any concentration (Figure 6, A). Similar results were obtained with primary amniotic fibroblasts; actinomycin D (1 μg/mL) induced significant (P < .001) apoptosis of these cells, which was even more pronounced than for the primary epithelial cells (Figure 5, A and B) because the endogenous apoptosis was lower in these cells (controls). When PBEF was added together with actinomycin D, this caused a dose-dependant and significant (P < .01) decrease in apoptosis at 100 ng/mL (Figure 6, B). There was no effect of PBEF alone on apoptosis.
Figure 6.
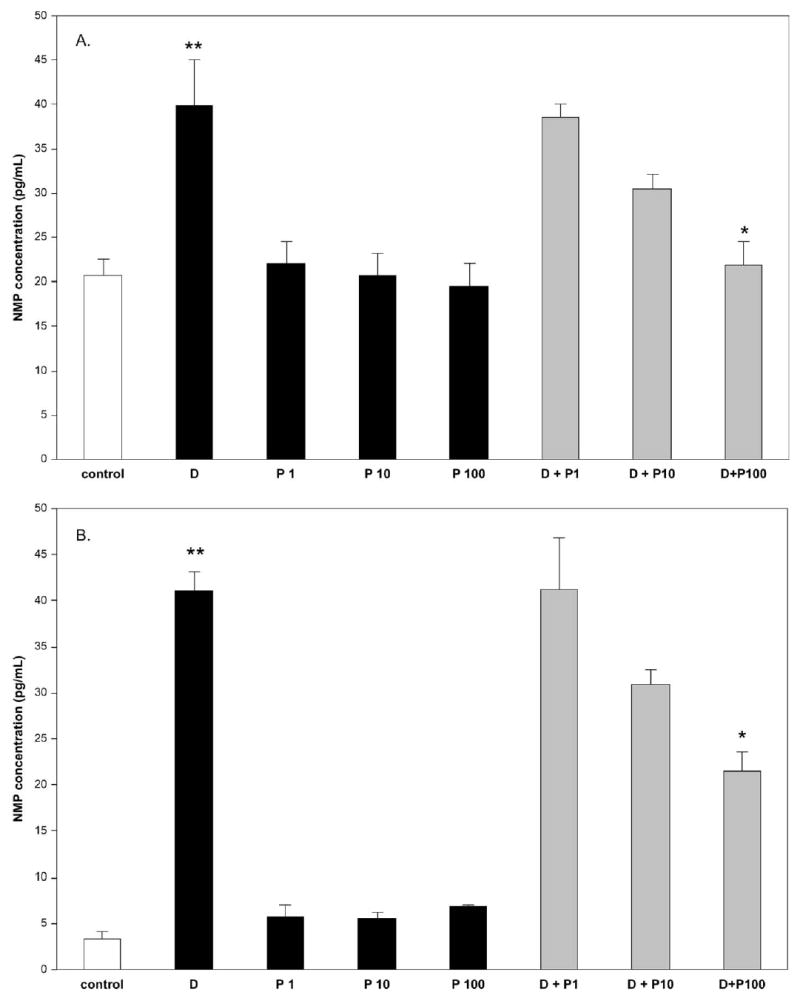
The effect of PBEF on late-stage apoptosis is shown in both primary amniotic epithelial cells (A) and fibroblasts (B) by measurement of the nuclear matrix protein (NMP) that was detected in the culture medium after treatment with actinomycin D. Cells were treated with actinomycin D (1 μg/mL) or treated with PBEF (1, 10, or 100 ng/mL), P1, P2, and P3, respectively, or with actinomycin D (1 μg/mL) together with each dose of PBEF (actinomycin D + P1, P2, or P3) for 72 hours. In both cell types, the actinomycin D caused a significant increase (**) (P < .001) in the amount of apoptosis; treatment with PBEF alone had no effect on the amount of apoptosis that was detected, whereas treatment with actinomycin D and PBEF showed a significant dose-response of the PBEF on the reduction of the apoptosis. This was significant at the highest dose of PBEF (100 ng/mL) for epithelial cells (P < .001) and for fibroblasts (P < .01), which was determined by the Kruskal-Wallis test and shown by*.
Comment
We have demonstrated that PBEF is secreted from the human amniotic epithelium and have shown it to have no effect on neutrophil chemotaxis but to have an antiapoptotic effect on both amniotic epithelial cells and fibroblasts. Recent studies have stated that PBEF is not a secreted protein because it is the intracellular enzyme nicotinamide phosphoribosyltransferase.2,7 In one of these studies, the investigators failed to detect any significant secretion of PBEF by either resting or anti-CD3–activated mouse T lymphocytes. However, a recent study shows that PBEF is secreted from human neutrophils. Conditioned media that were collected from neutrophils that were treated with lipopolysaccharide contained immunoreactive-secreted PBEF; this was shown to have a similar antiapoptotic effect on neutrophils, as did PBEF added to the cells.8 This study showed that PBEF is a secreted product of neutrophils and has antiapoptotic properties. In neither this study nor our study was it possible to give an absolute concentration of PBEF secreted by the cells, currently there is no PBEF standard by which to measure this. However, it appears that PBEF does not use any classic secretory pathway to get out of the cells,2 neither does it use the alternative secretory pathway described for IL-1β , a secreted protein that similarly lacks a signal peptide, but instead uses regulated exocytosis of pre-terminal endocytic vesicles to transport the cytosolic IL-1β from the cell.14 Thus, PBEF may use another yet unidentified secretory pathway to exit the cell. In any event, this secretion of PBEF does not preclude it being both an intracellular mediator of metabolism and a secreted cytokine with extracellular actions.
We have shown here that PBEF has no effect on the chemotaxis of neutrophils when compared with the effect of IL-8, a major chemotaxic and activating factor for neutrophils.15 The concentration of IL-8 used here was relevant physiologically and in the range that was reported recently in vivo in cervical mucus.16 We previously showed that distension causes an increase in the expression of both PBEF and IL-8 and that PBEF is able to induce the transcription of IL-8.5 Thus, although PBEF has no effect on chemotaxis, its induction of IL-8 would cause this. Chemotaxis is important in the setting of severe infection, a situation in which both PBEF and IL-8 are up-regulated in vivo in the fetal membranes.5
The antiapoptotic effect of PBEF on both amniotic epithelial cells and fibroblasts that has been shown here and on neutrophils in a recent study, in which PBEF was shown to prolong the survival of neutrophils in patients with clinical sepsis, acting by inhibiting constitutive neutrophil apoptosis,8 suggests that this is a highly conserved extracellular function of PBEF. The authors used the antisense oligonucleotide to block PBEF gene translation and showed it to abrogate the effect of inflammatory agents completely on prolonged neutrophil survival in clinical sepsis. The same mechanism of inhibiting constitutive apoptosis may also lead to the increased numbers of neutrophils (neutrophilia) that are observed in normal pregnancy.17 Although the number of neutrophils is increased, only mild neutrophil activation is normally observed; marked neutrophil activation is associated with pathogenesis (preeclampsia).18
Apoptosis may also play an important role in the fetal membranes. Apoptosis in the human amnion has been shown to be increased significantly by labor at term.19 The molecular mechanism underlying this was shown to involve the activation of caspases 3 and 8,20 which is significant because the inhibition of these 2 caspases has been shown to be the mechanism of the PBEF antiapoptotic activity in neutrophils.8 We have shown here that both early and late stages of apoptosis are inhibited by PBEF. This agrees with the study of PBEF action on neutrophils in which it was shown that PBEF inhibited the cleavage of caspase-8.8 Thus, PBEF may target early membrane-linked events that are associated with caspase-8 activation. In this study we have shown that the terminal event of apoptosis, measured by the nuclear matrix protein released on apoptosis, is affected significantly by PBEF in the presence of an apoptosis-inducing agent. Further dissection of the antiapoptotic action of PBEF on both amniotic epithelial cells and fibroblasts is needed.
These studies show that PBEF is indeed a secreted protein from the human amnion and has some of the more classic actions as a cytokine, despite of its lack of structural homology with other cytokines. Although both absolute concentrations of PBEF that are produced in the fetal membranes and the levels in amniotic fluid during pregnancy are unknown, it is likely that the local production and actions of PBEF are biologically relevant.5 These actions may be important in the protection of the human amniotic epithelium against apoptosis during the latter part of gestation, when the membranes become significantly stretched in vivo.21 We have shown that PBEF expression increased with the stretching of this tissue in vitro, but there was no effect on the apoptosis of these cells,3 which suggests that the PBEF may have been one factor that inhibited apoptosis. On the other hand, in the setting of infection, although PBEF has no direct effect on chemotaxis, its stimulation of IL-8, the major neutrophil attracting factor,15 may be important. Further studies are needed to clarify the mechanism of action of PBEF in both sterile distension of the fetal membranes and in chorioamnionitis.
Acknowledgments
We thank Mrs Sandra Yamamoto for the isolation and growth of human amnion cells, Amgen Inc for the rabbit anti-PBEF antibody that was used in this study, and Dr John Marshall for providing the sequences of his antisense and sense oligonucleotides before their publication.
Footnotes
Supported by National Institutes of Health grant HD-24314 and by grants to the University of Hawaii under the Research Centers in Minority Institutions Program of the National Center for Research Resources (P20RR 11091 and P20RR 16467) and a grant from the Hawaii Community Foundation (20031973).
References
- 1.Samal B, Sun Y, Stearns G, Xie C, Suggs S, McNiece I. Cloning and characterization of the cDNA encoding a novel human pre–B-cell colony–enhancing factor. Mol Cell Biol. 1994;14:1431–7. doi: 10.1128/mcb.14.2.1431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rongvaux A, Shea RJ, Mulks MH, Gigot D, Urbain J, Oberdan L, et al. Pre–B cell colony–enhancing factor, whose expression is up-regulated in activated lymphocytes, is a nicotinamide phosphoribosyltransferase, a cytosolic enzyme involved in NAD biosynthesis. Eur J Immunol. 2002;32:3225–34. doi: 10.1002/1521-4141(200211)32:11<3225::AID-IMMU3225>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 3.Nemeth E, Tashima LS, Yu Z, Bryant-Greenwood GD. Fetal membrane distention: I, differentially expressed genes regulated by acute distention in amniotic epithelial (WISH) cells. Am J Obstet Gynecol. 2000;182:50–9. doi: 10.1016/s0002-9378(00)70490-x. [DOI] [PubMed] [Google Scholar]
- 4.Ognjanovic S, Bao S, Yamamoto SY, Garibay-Tupas J, Samal B, Bryant-Greenwood GD. Genomic organization of the gene coding for human pre–B-cell colony–enhancing factor and expression in human fetal membranes. J Mol Endocrinol. 2001;26:107–17. doi: 10.1677/jme.0.0260107. [DOI] [PubMed] [Google Scholar]
- 5.Ognjanovic S, Bryant-Greenwood GD. Pre–B-cell colony–enhancing factor, a novel cytokine of human fetal membranes. Am J Obstet Gynecol. 2002;187:1051–8. doi: 10.1067/mob.2002.126295. [DOI] [PubMed] [Google Scholar]
- 6.Ognjanovic S, Tashima LS, Bryant-Greenwood GD. The effects of pre–B-cell colony enhancing factor on the fetal membranes by microarray analysis. Am J Obstet Gynecol. 2003;189:1187–95. doi: 10.1067/s0002-9378(03)00591-x. [DOI] [PubMed] [Google Scholar]
- 7.Kitani T, Okuno S, Fujisawa K. Growth phase–dependant changes in the subcellular localization of pre–B cell colony–enhancing factor. FEBS Lett. 2003;544:74–8. doi: 10.1016/s0014-5793(03)00476-9. [DOI] [PubMed] [Google Scholar]
- 8.Jia SH, Li Y, Parodo J, Kapus A, Fan L, Rotstein OD, et al. Pre–B-cell colony enhancing factor inhibits neutrophil apoptosis in experimental inflammation and clinical sepsis. J Clin Invest. 2004;113:1318–27. doi: 10.1172/JCI19930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol Reprod. 1996;55:1253–60. doi: 10.1095/biolreprod55.6.1253. [DOI] [PubMed] [Google Scholar]
- 10.Boyum A. Isolation of mononuclear cells and granulocytes from human peripheral blood. Scand J Clin Lab Invest. 1968;21:77–89. [PubMed] [Google Scholar]
- 11.Quan JM, Martin TR, Rosenberg GB, Foster DC, Whitemore T, Goodman RB. Antibodies against the N-terminus of IL-8 receptor A inhibit neutrophil chemotaxis. Biochem Biophys Res Commun. 1996;219:405–11. doi: 10.1006/bbrc.1996.0246. [DOI] [PubMed] [Google Scholar]
- 12.Harvath L, Brownson NE, Fields G, Skubitz APN. Laminin peptides stimulate human neutrophil motility. J Immunol. 1994;152:5447–56. [PubMed] [Google Scholar]
- 13.Frevert CW, Wong VA, Goodman RB, Goodwin R, Martin TR. Rapid fluorescence-based measurement of neutrophil migration in vitro. J Immunol Methods. 1998;213:41–52. doi: 10.1016/s0022-1759(98)00016-7. [DOI] [PubMed] [Google Scholar]
- 14.Andrei C, Dazzi C, Lotti L, Torrisi MR, Chimini G, Rubartelli A. The secretory route of the leaderless protein interleukin 1β involves exocytosis of endolysosome-related vesicles. Mol Biol Cell. 1999;10:1463–75. doi: 10.1091/mbc.10.5.1463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Baggiolini M, Walz A, Kunkel SL. Neutrophil activating peptide/interleukin-8 a novel cytokine that activates neutrophils. J Clin Invest. 1989;84:1045–9. doi: 10.1172/JCI114265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sakai M, Sasaki Y, Yoneda S, Kasahara T, Arai T, Okada M, et al. Elevated interleukin-8 in cervical mucus as an indicator for treatment to prevent premature birth and preterm prelabor rupture of membranes: a prospective study. Am J Reprod Immunol. 2004;51:220–5. doi: 10.1111/j.1600-0897.2004.00145.x. [DOI] [PubMed] [Google Scholar]
- 17.Redman CWG, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180:499–506. doi: 10.1016/s0002-9378(99)70239-5. [DOI] [PubMed] [Google Scholar]
- 18.van Dadelszen P, Watson RW, Noorwali F, Marshall JC, Parodo J, Farine D, et al. Maternal neutrophil apoptosis in normal pregnancy, preeclampsia, and normotensive intrauterine growth restriction. Am J Obstet Gynecol. 1999;181:408–14. doi: 10.1016/s0002-9378(99)70570-3. [DOI] [PubMed] [Google Scholar]
- 19.Hsu CD, Meaddough E, Basherra H, Harirah H, Lu LC. Increased apoptosis in human amnion is associated with labor at term. Am J Reprod Immunol. 2000;43:255–8. doi: 10.1111/j.8755-8920.2000.430502.x. [DOI] [PubMed] [Google Scholar]
- 20.Kumagai K, Otsuki Y, Ito Y, Shibata MA, Abe H, Ueki M. Apoptosis in the normal human amnion at term, independent Bcl-2 regulation and onset of labour. Mol Hum Reprod. 2001;7:681–9. doi: 10.1093/molehr/7.7.681. [DOI] [PubMed] [Google Scholar]
- 21.Millar LK, Stollberg J, DeBuque L, Bryant-Greenwood GD. Fetal membrane distention: determination of the intrauterine surface area and distention of the fetal membranes preterm and at term. Am J Obstet Gynecol. 2000;182:128–34. doi: 10.1016/s0002-9378(00)70501-1. [DOI] [PubMed] [Google Scholar]


