Abstract
Early placental insulin-like protein (INSL4 or EPIL) is a member of the insulin superfamily of hormones which is highly expressed in the placenta. We have confirmed this at term and shown it to be expressed by the maternal decidua. Although an abundance of locally acting growth factors are produced within the uterus during pregnancy, we hypothesized that INSL4 plays an important role in fetal and placental growth. We have demonstrated with cell lines and primary cells that it has a growth inhibitory effect by causing apoptosis and loss of cell viability. We used primary amniotic epithelial cells for flow cytometry to show that INSL4 caused apoptosis, which was dose related and significant (p<0.05) at 50ng/ml. This was confirmed by measurement of the nuclear matrix protein in the media. In comparison, relaxin treatment (up to 200ng/ml) had no effect on apoptosis. The addition of INSL4 (3-30ng/ml) also caused a loss of cell viability, although it had no effect on the numbers of cells at different phases of the cell cycle.
Placental apoptosis is an important process in both normal placental development and in fetal growth restriction. Therefore an in vivo clinical correlate was sought in fraternal twins exhibiting discordant growth. Expression of INSL4 was doubled in the placenta of the growth restricted twin compared to the normally grown sibling, suggesting it may be linked to a higher level of apoptosis and loss of cell viability, and may therefore contribute to fetal growth restriction.
Keywords: early placental insulin-like peptide, EPIL, insulin-like 4, INSL4, placenta, apoptosis, fetal growth restriction
INTRODUCTION
The synchronization of the growth of the fetus, uterus and placenta appears to be coordinated by key growth factors as well as substrate bioavailability. In recent years, the importance of the insulin-like growth factors, IGF1 and IGF2, has become clear. Cord blood levels of IGF1 and IGF2 correlate with birth and placental weights [1] and their levels are significantly lower in growth restricted fetuses compared to normally grown controls [2,3]. A chimaeric mouse model with disruption of one of the Igf2 alleles had progeny which were smaller than average [4]. We have recently shown that relaxin, a member of the insulin hormone superfamily, acts as a growth factor for an amniotic epithelial-like cell line (WISH), and possibly as an autocrine/paracrine hormone of the human fetal membranes. We demonstrated that relaxin in vitro probably causes the proliferation of WISH cells by increasing the transcription of IGF2 [5].
The early placental insulin-like peptide, (EPIL or INSL4) is also a member of the insulin superfamily, it has the most homology with the human relaxins, RLN1 and RLN2 (44% and 43% respectively), and only 15% homology to insulin [6, 7]. It was initially identified from a subtracted cDNA library of a first trimester placenta [6, 8], and was shown to be most highly expressed in embryonic and trophoblastic tissues [9]. Indeed its high level of expression in the placental syncytiotrophoblast early in gestation suggested it to be of importance in fetal and placental growth and development. The INSL4 gene is comprised of two exons and one intron, similar to the other members of its superfamily. The clustering of this gene with the two human relaxin genes RLN1 and RLN2 on the same chromosome at 9p24 [10] suggests that the gene for INSL4 and the RLN1 gene resulted from late gene duplication events. The INSL4 gene is only expressed in higher primates, probably after the divergence of the New World and Old World monkeys [7]. It has recently been shown that a human endogenous retrovirus element is inserted into the human INSL4 gene promoter. Its placental specific expression is mediated by the 3′ LTR of the retroviral element, suggesting that this ancient retroviral infection may have been a major event for the functional evolution of the human placenta [11].
The INSL4 protein appears to be unlike relaxin or insulin, which have their mature peptides fully processed and therefore lack the connecting peptides. The IGFs on the other hand, remain completely unprocessed in their mature forms. Because the INSL4 protein only has dibasic recognition sites for putative enzyme cleavage between the C and A domains, two peptide chains of 13kDa and 4kDa would be produced [8]. However, it is currently unknown how this peptide is processed in vivo. It has been shown that the levels of the proINSL4 protein decrease in amniotic fluid with advancing gestation, at the same time as its concentrations in serum rise. The pattern of proINSL4 levels in amniotic fluid in both normal and chromosomally abnormal pregnancies correlate strongly with the levels of chorionic gonadotrophin and its free subunits, suggesting that the production of these hormones by the syncytiotrophoblast may be controlled by a common regulatory pathway [12,13]. However, synthetic INSL4 has no hydrophobic core and a lack of helical structure at physiological pH [14]. No specific target tissue has been identified and it lacks binding activity to the relaxin receptor (LGR7), its splice variant, or to the INSL3 receptor (LGR8) [7]. Indeed, no biological function has yet been ascribed to this protein. Because of its structural homologies with other hormones involved in growth regulation, we therefore sought biological action(s) for INSL4 in the human amnion and placenta and compared them to the effects of human relaxin 2 (RLN2). At the same time, an in vivo correlate was sought, to link any biological activity with a clinically defined obstetrical problem. This was important because inappropriate fetal growth is a significant risk factor for preterm birth [15,16]. Both large and small for gestational age infants have a 2 to 3-fold increased risk of preterm delivery when compared to normally grown infants [16]. The greater the fetal growth impairment, the higher the risk of preterm birth. Infants with severe growth impairment have a six-fold increased rate of preterm birth secondary to preterm premature rupture of membranes compared to normally grown newborns [17].
MATERIALS AND METHODS
Hormones
INSL4 was chemically synthesized with an insulin-like disulfide bonding pattern as previously described [14]. Because of the lack of basic amino acids at the B/C peptide junction, it was synthesized with a 36 amino acid residue B chain, with the C-terminal pentapeptide omitted [14]. Recombinant human relaxin 2 was a generous gift from BAS Medical Inc. (San Mateo, CA) and recombinant IGF2 was obtained from Bachem Inc. (Torrance, CA).
Patients, tissues and cell culture
Fetal membranes and placentas were collected from Kapiolani Medical Center for Women and Children (Honolulu, Hawaii, USA) with informed consent and approval from the Institutional Review Board. For the study of INSL4 gene expression in the placenta by RT-PCR, a tissue was collected after elective Cesarean section at term (n=1). A sample of placental trophoblast was taken from the center of the tissue avoiding the chorionic and basal plates, and the full thickness fetal membrane was also collected. For the quantitative real-time PCR, placental trophoblast (n=4) was similarly collected and the membranes separated (n=4), the amnion was carefully stripped from the chorio-decidua and the decidua scraped from the chorion. None of these tissues had any signs of histological chorioamnionitis. The placental distribution of INSL4 expression was studied by collecting term placental samples from both Cesarean sections prior to labor (n=5) and NSD (n=5) from the chorionic and basal plates and mid-way between these two sites, trophoblast was collected both from a lobe adjacent to the umbilical cord and a lobe at the edge of the placenta. These patients had no clinical evidence of infection, but histological chorioamnionitis was not excluded. For an in vivo model of fetal growth restriction, placental samples (n=3) and full thickness membrane (n=4) were collected from patients delivering preterm discordant twins (32-37 weeks gestational age) by elective Cesarean section prior to the onset of labor, the demographic data for these patients is shown in Table 1. These were chosen over singleton pregnancies because one twin of each pair was of normal size, and was therefore a perfect control for the growth-restricted twin. The intrauterine environment was otherwise identical and decreased the normal variability observed when placentas from singleton pregnancies are used. In addition, gestational length was identical for each twin in a pair. Discordant twins differ by 25% in weight, and the 5 pairs used here had a mean discordancy of 33%. The samples used were either monoamniotic or diamniotic/dichorionic. Samples were taken from both the growth restricted and normally grown placentas by harvesting villous trophoblast as a cross-section of tissue from the peripheral edge. None of these patients had any clinical or histological evidence of infection. Samples were collected into liquid nitrogen and stored at −80°C until used, except those used for INSL4 gene expression throughout the placenta, which were stored at 4°C in RNAlater for several days before use. For the primary amnion cell cultures, the fetal membranes were cut from the placentas of women delivering at term by Cesarean section (n=7). The amniotic epithelial cells and fibroblasts were isolated by two methods as previously described [18, 19]. These were compared and the yield of fibroblasts was found to be considerably greater with the Casey and MacDonald method [19]. In addition, the contamination of each cell type with the other was lower (~2% fibroblasts contaminating the epithelial cells and ~10% epithelial cells in the fibroblasts), assessed by immunocytochemical staining for cytokeratin (epithelial cells) and vimentin (mesenchymal cells). This method [19] was therefore used for further studies. The Mann-Whitney nonparametric two-tailed test was used for analysis of gene expression in the fetal membranes and placentas, and the results presented as mean ± SD.
TABLE 1.
Demographic data of normally grown and growth restricted infants (n=5 pairs)
| Gestational age (weeks) | Normally grown twina | Growth restricted twina |
|---|---|---|
| 37c | 2863 | 2126 |
| 36c | 1942 | 1508 |
| 36b,c | 2191 | 1600 |
| 34b,c | 1911 | 1270 |
| 32b | 2082 | 883 |
Birth weight (grams)
Used for placentabor membranec
Culture of cell lines: WISH and JAR cells and their treatment
Two cell lines were used; JAR cells (ATCC HTB-144) representative of the placenta and human amnion-derived WISH (ATCC CCL-25) cells, representative of the fetal membranes. Both were obtained from the American Type Tissue Culture Collection (Manassas, VA). The WISH cells were grown in Dulbecco Modified Eagle medium (DMEM):F12 supplemented with 10% fetal bovine serum (FBS, Invitrogen, Carlsbad, CA), penicillin (100 U/ml) and streptomycin (100 μg/ml). JAR cells were grown in RPMI 1640 medium supplemented with 10% FBS and antibiotics at the same concentrations as used for WISH cells. Both cell types were incubated at 37°C in 5% carbon dioxide, 95% air. When at 70% to 80% confluency, they were either dispersed with 0.25% trypsin-1.0mmol/L EDTA and plated as described for each experiment or treated as described below for flow cytometry.
Cell proliferation assays
WISH and JAR cells were plated into 96-well culture plates at a density of 5,000 cells per 200μl growth medium, supplemented with 5% FBS. After the cells attached (48h), they were washed with minimal media (0.25% FBS in DMEM:F12) and treated with either INSL4 (3, 10, 30 ng/ml) or IGF2 (30 ng/ml) for 5 days. The dose of IGF2 was predetermined [5]. The INSL4 and IGF2 were diluted in minimal media, which was replaced after 48h. Cells incubated in media alone were used as controls. The number of viable cells was measured with the CellTiter96 Aqueous One Solution kit (Promega, Madison, WI) as per the manufacturer’s instructions (n=4 for both cell lines). The One Solution reagent was added to the culture media and the cells incubated for 30 min at 37°C in 5% carbon dioxide and 95% air, after which the absorbance at 490 nm was read in a 96-well microplate reader (Molecular Devices, Sunnyvale, CA). Statistical analysis was performed by the two-tailed Mann-Whitney test, and expressed as percentage of control value ± SD.
Cell cycle analysis
WISH cells were plated into 100mm dishes at a density of 2×106 cells per 10ml medium, supplemented with growth media (5% FBS in DMEM:F12) and grown for 48h. The media was removed, the cells washed in Hanks Balanced Salt Solution (HBSS) and reincubated for synchronization for 24h in minimal media (0.25% FBS in DMEM:F12). They were then treated with INSL4 (30ng/ml) or staurosporine (100nM) (Sigma, St. Louis, MO) as a positive control [20] for 48h in growth medium. Cells incubated in growth media alone acted as negative controls. At the end of the incubation they were counted and 2×106 cells thoroughly resuspended in cold PBS, fixed in 70% ethanol and placed on ice for at least 2h. These cells were pelleted, resuspended in PBS, allowed to sit for 60 secs and repelleted. The supernatants were decanted and the pellets resuspended in propidium iodide (PI) staining solution (PI 2%, DNase-free RNase 20%, Triton X-100 1% in PBS) and incubated for 30 min at room temperature in the dark. Fluorescence was measured by flow cytometry with a Beckman Coulter Epics XL instrument with a 488nm argon laser and 10,000 cells per sample were counted, cell doublets were gated out. The flow cytometry data was analyzed with the Multicycle Program (Phoenix Flow Systems, San Diego, CA) to determine the percentage of cells in G1/G0, S and G2/M phases of cell growth. Four experiments were performed at different times. Statistical analysis used the two tailed Mann-Whitney test and expressed as average percentage of total cells ± SD.
Analysis of apoptosis
Several commercial reagents were tested in order to determine the optimal method of detachment of the primary amniotic epithelial cells for flow cytometry. Amniotic epithelial cells were seeded into 6-well plates and grown for 7-11 days in Ham’s F12/DMEM with 10% FBS. At 90% confluence, the media was removed and the cells washed with HBSS. They were either scraped from the dish with a rubber policeman, incubated with either 750 μl Accutase (Innovative Cell Technologies, San Diego, CA) or Accumax (Innovative Cell Technologies) for 10 min at 37° C. Other cells were treated with Sodium Citrate-0.135M KCl solution for 5 min at 37° C (www.medicine.uiowa.edu/flowcytometry/citric_saline.html) or Cell-Stripper (Mediatech, Hearndon, VA) for 5 min at 37° C. Accutase preserved the viability of the detached cells, which was so pronounced in comparison to the other treatments that it was used for all further experiments. Staurosporine was used to induce apoptosis as previously described [20]. In order to determine its correct concentration for apoptosis induction, amniotic epithelial cells and fibroblasts (n=3, from different patients) were treated with a range of concentrations (25, 50 or 100 nM) for 12 (Fig. 1) and 24 h (not shown), and analyzed by flow cytometry. For the epithelial cells, both 50 and 100 nM staurosporine caused an increase in the numbers of apoptotic cells when compared to the controls and these concentrations were used for further studies (Fig.1A). However, staurosporine failed to cause apoptosis in fibroblasts (Fig. 1B), therefore all further experiments were conducted with epithelial cells.
FIG.1.
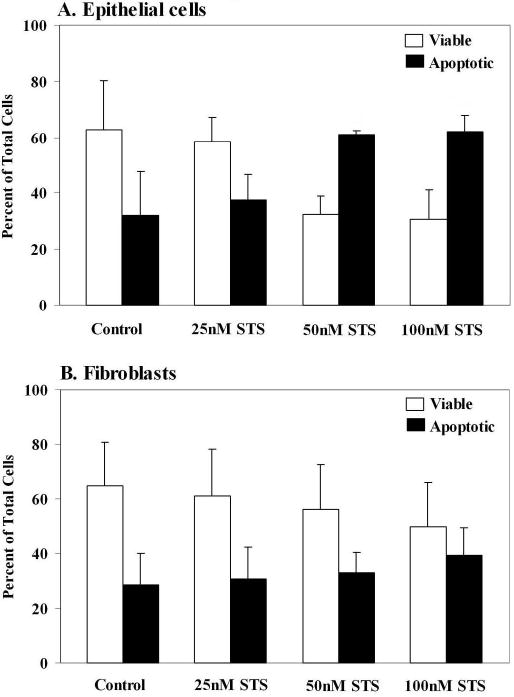
Determination of the effect of staurosporine (STS) treatment (one well per dose) on induction of apoptosis by flow cytometry in A. primary amniotic epithelial cells and B. fibroblasts (n=3 from different patients). The epithelial cells showed increased apoptosis with 50 and 100nM STS, compared to the controls. There was no effect of STS on the apoptosis of fibroblasts. Results are shown as means ± SD.
The effect of INSL4 on apoptosis was compared to that of 100nM staurosporine, which caused maximal apoptosis. Primary amniotic epithelial cells (n= 4 different patients), were treated with INSL4 (5, 25, 50ng/ml) or staurosporine (100nM) in 1.5 ml treatment media (Ham’s F12/DME with no FBS or antibiotics) for 24 h at 37°C. The controls were grown in media alone. After treatment, media were removed and centrifuged to pellet floating cells. The remaining cells on the plate were washed with HBSS and added to the same tubes. Accutase (750μl) was added to each well and the plates incubated at 37°C for 10 min. The cells were then detached by gentle scraping and pooled with the floating cells. They were centrifuged and washed with 1× Annexin Binding Buffer (Molecular Probes, Eugene, OR), re-centrifuged and the supernatant removed. Apoptosis was detected with the Vybrant Apoptosis Assay (Molecular Probes) according to the manufacturer’s protocol. This assay is based upon the binding by the apoptotic cells of Annexin V conjugated to the fluorescent dye, Alexa Fluor 488. Propidium iodide (PI) was used to monitor necrosis as these cells stain both orange (PI) and green (Alexa Fluor), while viable cells exclude both dyes. Alexa Fluor/PI staining solution (200μl) (Molecular Probes) was prepared as directed by the manufacturer and added to each pellet. The cells were resuspended, and incubated for 15 min at room temperature in the dark. 1× binding buffer (800μl) was then added to each tube and placed on ice. Cell fluorescence was measured by flow cytometry using a Beckman Coulter Epics XL with a 488 argon laser. Statistical analysis was performed using the Mann-Whitney two-tailed test, and expressed as means ± SD.
The effects of INSL4 on late-stage apoptosis was also measured from the amount of nuclear matrix protein present in the media from these experiments (n=4). This protein is only released into the medium by apoptotic and necrotic cells. The protein was measured using an ELISA assay (Calbiochem, Temecula, CA). Significance was determined by the Mann-Whitney two-tailed test, and expressed as means ± SD.
To study the effects of relaxin (RLN2) on apoptosis, amniotic epithelial cells (n=7, different patients), were incubated with 50nM staurosporine either alone or together with 200ng/ml human relaxin for 12h in treatment media. Controls included media alone or relaxin alone. Following treatment, the cells were detached with Accutase. Floating and adherent cells were combined and pelleted by centrifugation as described above. Apoptosis was measured using the Vybrant Apoptosis Kit (Molecular Probes), following the manufacturer’s protocol and analyzed by flow cytometry as above.
Isolation of total RNA, RT-PCR and quantitative real-time PCR
RNA was isolated from samples of separated amnion, chorion, decidua and placenta as previously described [5]. For the regionalization of expression in the placenta, RNA was isolated using the RNeasy kit according to the manufacturer’s instructions (Qiagen, Valencia, CA). For the twin placentas and membranes, RNA was extracted using either the RNeasy kit or TRIZOL (Invitrogen). RNA from WISH and JAR cells was extracted using TRIZOL. All RNA samples used for quantitative real-time PCR were treated with either DNA-Free (Ambion, Austin, TX) or RNase-free DNase (Qiagen) to remove any contamination with genomic DNA. For RT-PCR, samples of total RNA (2μg) obtained from WISH and JAR cells, fetal membranes and placenta were reverse transcribed using Moloney Murine Leukemia Virus and random hexamers according to the manufacturer’s protocol (Applied Biosystems, Foster City, CA). PCR amplification was performed using specific primers for INSL4 (Genbank accession number: L34838) (INSL4 forward primer: 5′AACTCCTTAGAGAAAGCCTAGCA3′ reverse primer: 5′TCGTACCTAAGGCTTGTCCATCT3′). DNA was denatured for 10min at 95°C followed by 40 cycles of 95°C for 15s, 49°C for 15s and 72°C for 30s. A final extension step was carried out for 10min. The products were visualized by gel electrophoresis.
Quantitative real-time PCR was performed using an iCycler (Bio-Rad Laboratories, Inc., Hercules, CA) or an Opticon (MJ Research, Reno, NV). Specific primers (300nM each) were made by Integrated DNA Technologies, Inc. (Coralville, IA) and used in conjunction with QuantiTech SYBR Green PCR kit (Qiagen) for the study of INSL4 expression in the amnion, chorion, decidua and 280 placenta (forward primer: 5′GCCTGAGAAGACATTCACCA3′, reverse primer: 5′TCGTACCTAAGGCTTGTCCA3′). A melting curve was performed at the end of the PCR reaction to confirm a single product. A FAM-labeled LUX (light upon extension) primer and probe set for INSL4 were designed by Invitrogen and used for quantitation of INSL4 expression in different areas of the placenta (LUX 285 probe: 5′GAGCTGTTCAGACAGTGGTTTCTTCAGCTC3′, primer: 5′GAGGGTGGCTGCTGGAATCT3′). For the iCycler real-time PCR with SYBR green, DNA samples were amplified for 1 cycle at 95 ° C for 15 min followed by 40 cycles of 95 °C for 15 sec, 60° C for 30 sec and 72° C for 30 sec. For the Opticon with SYBR green, DNA samples were amplified for 1 cycle at 95 ° C for 15 min followed by 40 cycles of 95° C for 15 sec, 60° C for 15 sec and 72° C for 20 sec. PCR using the LUX probe and primer with Amplitaq Gold (Applied Biosystems) was carried out on the Opticon using these conditions: DNA samples were amplified for 1 cycle at 95° C for 10 min followed by 40 cycles of 95° C for 15 sec and 60° for 1 min. All samples were run in triplicate and a dilution series of an INSL4 cDNA probe (583bp) in PCR 2.1 vector (Invitrogen) was used as a standard in all real-time PCR reactions. Statistical analysis was performed with the Mann-Whitney two-tailed test and expressed as means ± SD.
RESULTS
Expression of the INSL4 gene was seen as a strong single band of 192bp in both the WISH and JAR cells and in the term placenta (Fig.2A, lanes 1, 4 and 3 respectively), but with these primers was detectable only as a very faint band in the full thickness fetal membranes (Fig.2A, lane 2). The PCR control (Fig.2A, lane 5) was negative. Quantitative real-time PCR on separated placenta, amnion, chorion and decidua collected at term Cesarean section prior to labor, showed a significantly higher level of INSL4 expression in the placenta (p<0.05) compared to the amnion, chorion or decidua. For the membranes, the maternal decidua had the highest expression followed by the amnion and chorion (Fig.2B). In order to determine if the expression of the INSL4 gene showed any pattern throughout the placenta, quantitative real-time PCR was used to compare its expression in the central area near the umbilical cord and at its periphery, as well as at three levels of thickness at each of these sites. This was performed on placentas obtained both before and after NSD. There was no significant difference at the different levels of thickness at either site in either Cesarean section or NSD samples. However, when the expression at each level in the central area was compared to that at the periphery, there was significantly greater expression of INSL4 at the periphery of the placenta in the basal plate (p<0.02), the trophoblast (p<0.03) and the chorionic plate (p<0.03). Shown for the placentas obtained at Cesarean section prior to labor (Fig.3). Similar significant differences were also obtained in placentas after NSD (data not shown). These were basal plate (p<0.03), trophoblast (p<0.008) and chorionic plate (p<0.03). Therefore INSL4 gene expression is predominantly at the periphery of the placenta.
FIG.2.
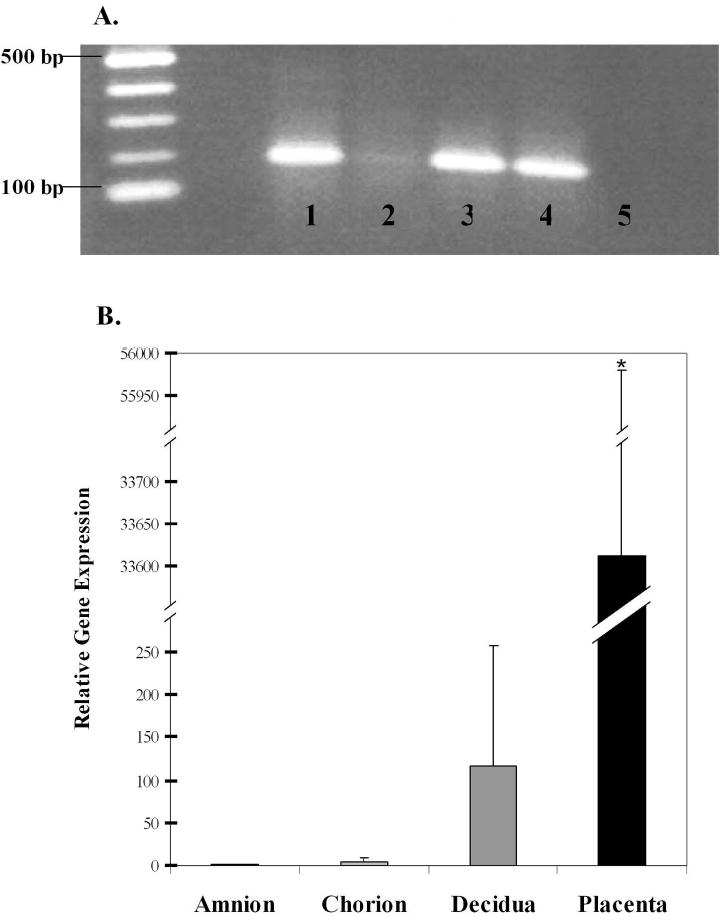
INSL4 gene expression by RT-PCR, shown in (A) as a single band of 192bp in cell lines and tissues. Lane 1: amniotic epithelial-like WISH cells, Lane 2: full thickness fetal membrane, Lane 3: placental trophoblast, Lane 4: choriocarcinoma JAR cells, Lane 5: negative control. (B) INSL4 expression in tissues by quantitative real-time PCR, shown as gene expression relative to the amnion. Tissues were all collected at term before labor, placenta (n=5), amnion, chorion and decidua (n=4). Means ± SD, asterisk indicates significantly more INSL4 expressed (p<0.05) in the placenta compared to the amnion, chorion or decidua.
FIG.3.
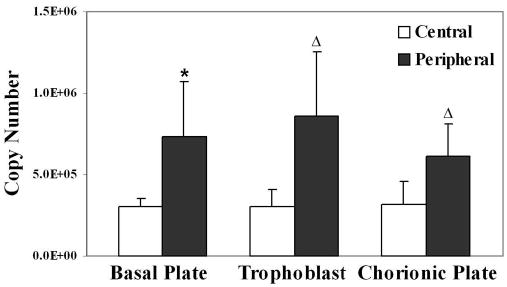
Expression of INSL4 by quantitative real-time PCR in different regions of the term placenta before labor (n=5) Tissue was collected from the central area near the umbilical cord and at the placental periphery, at three levels of thickness, the basal plate, trophoblast and chorionic plate. Results are expressed as means ± SD of INSL4 copy number. There was significantly greater expression of INSL4 at the periphery of the placenta in the basal plate (p<0.02) (asterisk), trophoblast (p<0.03) and chorionic plate (p<0.03) (triangles) compared to the central region of each.
The treatment of primary amniotic epithelial cells with staurosporine caused significant apoptosis (p<0.05) measured by flow cytometry (Fig.4 A, B and D). Their treatment with INSL4 caused a dose-related induction of apoptosis, almost reaching significance at 25ng/ml (p=0.057) and becoming significant at 50ng/ml (p<0.05) (Fig.4C and D). The cytograms from the flow cytometric analysis (Fig.4A, B and C) show that most of the control cells were viable. The cells excluded both dyes and appeared in the lower left quadrant (Fig. 4A). Treatment with staurosporine (100nM) induced apoptosis, as indicated by a large number of cells staining with Alexa Fluor 488 and moving to the lower right quadrant (Fig. 4B). Treatment with INSL4 also increased the numbers of apoptotic cells (Fig.4C), although not as many of the cells shifted as with the staurosporine treatment (Fig.4B). However, the INSL4 also caused an increase in the numbers of necrotic cells which stained with both reagents, shifting them to the upper right quadrant (Fig.4C), this result was not significant. The effect of INSL4 on apoptosis was also reflected in the increased nuclear matrix protein concentrations in the media (Fig. 4E), which was significant with both 25 (p<0.05) and 50 ng/ml (p<0.05) concentrations of INSL4.
FIG.4.
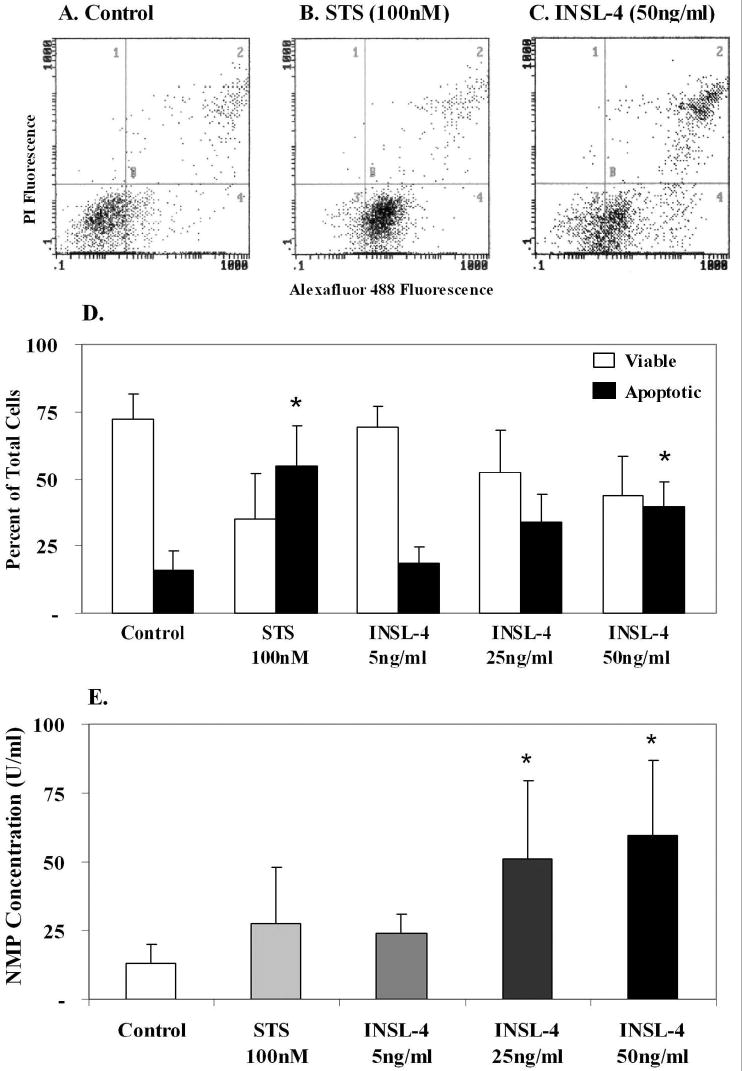
Effect of treatment of primary amniotic epithelial cells with INSL4 for 24 h (n=4, different patients). Cytograms of flow cytometric analysis: A. control, untreated cells, B. staurosporine (STS) induced apoptosis C. INSL4 treatment, showing that INSL4 also induced apoptosis and necrosis. D. quantitation of the flow cytometry data means ± SD, showing a dose-related effect of INSL4 on induction of apoptosis. Asterisks show STS significantly (p<0.05) induced apoptosis compared to the controls, INSL4 (50ng/ml) significantly induced apoptosis (p<0.05). E. Nuclear matrix protein (NMP) measurement in the medium of the experiments shown in D as means ± SD, reflect the effect of INSL4 on causing apoptosis and necrosis. Asterisks show significantly increased (p<0.05) NMP compared to the controls.
A similar study was carried out with human relaxin, but unlike INSL4, it had no effect on the numbers of apoptotic cells. The cytograms show increased numbers of apoptotic cells induced by treatment with staurosporine (Fig.5B) compared to the control (Fig.5A), and lack of an effect of relaxin (200ng/ml) (Fig.5C). However, when the same concentrations of staurosporine and relaxin were used together, relaxin reduced the numbers of apoptotic cells from 61% to 46% (Fig.5D), the quantitative results are shown in Fig.5E. Although this effect of relaxin failed to reach statistical significance, the results were in marked contrast to the effects of INSL4 shown in Fig.4. Thus, INSL4 and relaxin appear to have opposite effects on the apoptosis of amniotic epithelial cells, INSL4 inducing apoptosis and relaxin protecting the cells from an apoptotic stimulus.
FIG.5.
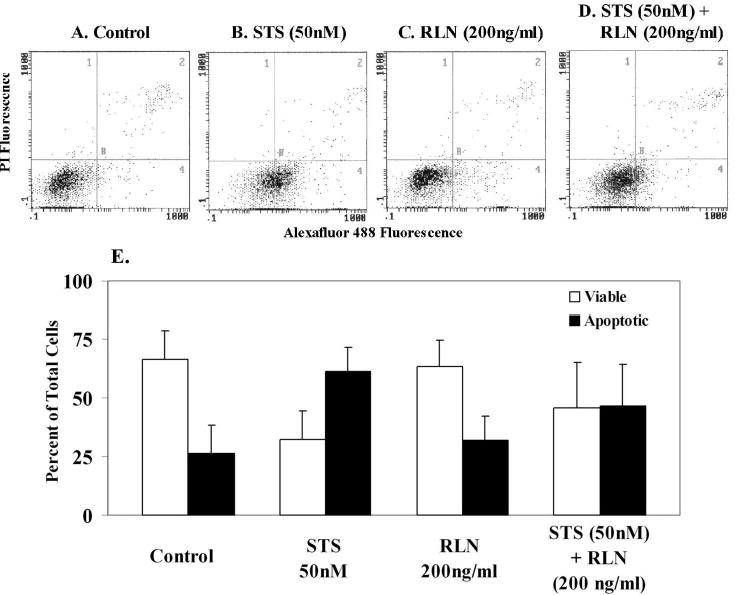
Effect of relaxin treatment (12 h) on the apoptosis of primary amniotic epithelial cells (n=7 different patients). Cytograms of flow cytometric analysis: A. control, untreated cells, B. staurosporine (STS) induced apoptosis C. relaxin (RLN2) treatment had no effect on the numbers of apoptotic cells D. STS and RLN2 together. RLN2 reduced the numbers of apoptotic cells from 61% to 46% (not significant). E. means ± SD of the flow cytometry data shown in A-D.
Because of the apoptotic effect of INSL4 in these studies, we sought an effect on the proliferation or number of viable WISH and JAR cells (representative of the amnion and placenta respectively). For these experiments, IGF2 (30ng/ml) was used as a positive control to induce (p<0.05) the proliferation of WISH cells (Table 2). Treatment with INSL4 (30ng/ml) significantly reduced (p<0.05) the numbers of WISH cells compared to the control. Treatment with INSL4 at 3 and 10ng/ml significantly reduced (p<0.05) the numbers of JAR cells (Table 2) compared to the control. The effect of INSL4 was therefore more pronounced on the placental JAR cells than on the amniotic-epithelial derived WISH cells. These results led us to seek an effect of INSL4 on the cell cycle using staurosporine as a positive control (Table 3). Staurosporine had a significant effect on the cell cycle (p<0.05) as expected, arresting cells in G2/M [20]. Treatment with INSL4 had no effect on the numbers of cells at the different stages of the cell cycle (Table 3).
TABLE 2.
The effects of INSL-4 on the viability of WISH and JAR cells.a
| Treatment | WISH | JAR |
|---|---|---|
| IGF-II (30ng/ml) | 154.4 ±23.9b | 104.1 ± 6.9 |
| INSL-4 (3ng/ml) | 90.3 ± 14.6 | 94.1 ± 6.2b |
| INSL-4 (10ng/ml) | 91.6 ± 14.9 | 88.4 ± 9.5b |
| INSL-4 (30ng/ml) | 81.3 ± 14.2b | 96.6 ± 13.9 |
values as % of control ± SD, n=4 for both WISH and JAR
p<0.05 compared to control
TABLE 3.
Cell cycle analysis of amniotic epithelial (WISH) cells.a
| Phase of CellCycle
|
|||
|---|---|---|---|
| Treatment | G1/G0 | S | G2/M |
| Control | 67.8 ± 2.4 | 24.1 ± 1.8 | 8.2 ± 1.0 |
| INSL-4 (30ng/ml) | 67.1 ± 2.9 | 24.5 ± 2.9 | 8.4 ± 0.8 |
| STS (100nM) | 7.2 ± 12.4b | 14.3 ± 4.6b | 78.5 ± 15.4b |
values presented as average % of total cells ± SD, n=4
p<0.05 compared to control
We sought an in vivo correlate of this in vitro data and measured the expression of INSL4 in the placentas and fetal membranes of discordant twins. INSL4 gene expression was more than two-fold greater in the placentas of growth restricted twins compared to normally grown twins (Fig. 6A), not reaching statistical significance due to the small sample size of these rare tissues. In contrast, INSL4 gene expression in the full thickness fetal membranes was similar in the growth restricted and normally grown twins (Fig. 6B).
FIG.6.

Expression of the INSL4 gene by quantitative real-time PCR in A. placentas (n=3) and B. fetal membranes (n=4) obtained from discordant twins, one twin with normal growth and the other with growth restriction (IUGR). Means ± SD shown, there was 2-fold more INSL4 expressed in the placentas of the IUGR twins (not significant).
DISCUSSION
In this study we show that INSL4 is produced by both the placenta and to a much lesser extent by the maternal decidua at term and that it probably acts locally to inhibit growth by causing apoptosis. It has been shown previously that INSL4 gene expression is highest in the syncytiotrophoblast, with less in the cytotrophoblast, villous stroma and intermediate trophoblast [9]. Our demonstration that there is significantly more INSL4 expressed in the periphery of the placenta in the basal plate, trophoblast and chorionic plate, both before and after spontaneous labor and delivery is important. This further suggests that INSL4 is associated with increased apoptosis and necrosis, as the normal human placenta shows 90% of infarctions at its margin [21]. The inhibitory effects of IN SL4 on growth are also in agreement with a recent study showing that a subclone of the SKBR3 breast cancer cell line with high levels of INSL4 expression had a much lower rate of growth than that of the parental cell line [22]. Together these observations suggest that INSL4 may be produced to both counter-balance rapid growth and to decrease growth as required. An abundance of growth stimulating peptides is produced by the intrauterine tissues. Several of these, like INSL4, are members of the insulin superfamily of hormones [2, 3, 5]. We compared the effects of relaxin and INSL4 on primary cells and showed that the two peptides had markedly different effects on apoptosis. Relaxin alone had no effect, but was mildly protective against apoptosis when used together with an apoptosis-inducing agent, INSL4 caused apoptosis. INSL4 therefore appears to be unique in this family of hormones in its action as an apoptotic agent/growth inhibitor.
Unfortunately, little is known about the structure of endogenous INSL4 and even less is known about its receptor or mode of action. Structurally, the native peptide may be significantly longer in length than the synthetic peptide used in this study [7, 14]. While the INSL4 molecule indeed appeared “structureless” in solution by CD spectroscopy criteria [14], such a study is no substitute for biological activity. Therefore, we believe that the results presented here supersede the idea that INSL4 is structureless and therefore has no biological function. A similar synthetic two-chain peptide corresponding to the predicted primary structure of INSL4 was recently shown to lack the ability to interact with LGR7 and LGR8. These leucine-rich repeat G-protein coupled receptor family members bind relaxin and INSL3 respectively [7]. It is therefore likely that INSL4 has its own receptor, which may or may not be a member of the same receptor family as LGR7 and LGR8. However, our study suggests that the synthetic peptide used was indeed able to interact with its receptor to cause a biological effect.
Placental apoptosis is an important process in normal placental development and increases as pregnancy advances [23]. An increased rate of apoptosis has been associated with placental and fetal growth restriction in singleton and twin pregnancies [24, 25]. We used flow cytometry and a cell proliferation assay to show that INSL4 affects the numbers of viable cells by causing apoptosis without affecting the cell cycle. Although the flow cytometry studies used only primary amniotic epithelial cells or an amniotic epithelial-like cell line (WISH), the cell proliferation study used both WISH cells and a placenta-derived cell line (JAR). INSL4 had the same effect on cell proliferation in both cell lines. This suggests that INSL4 plays a similar role in growth in both the placenta and the amniotic epithelium. In the apoptosis study, INSL4 also slightly increased the numbers of necrotic cells, but this was not statistically significant.
We sought an in vivo clinical correlate for this finding by using tissues from fraternal twins with major discordant growth and showed twice the level of INSL4 gene expression in the placentas of growth restricted compared to the normally grown twins. This suggests INSL4 may be linked to the increased apoptosis in these tissues. There was no difference in its expression in the fetal membranes of these patients, suggesting that in vivo its effects may be greater in the placenta than in the membranes. Unfortunately this study failed to reach significance because of the marked variability in expression in different patients and the small number of samples available. Discordant twin samples were optimal for study because of the controlled maternal environment for both the pathological twin and its normally grown control. We additionally excluded tissues from patients in labor or with clinical or histological evidence of chorioamnionitis. Such tissues are rarely available, limiting our sample size.
It is known that gene expression can vary in the normal placenta, both within and between different sites [25]. We sampled various areas of the placenta for variation in the expression of INSL4 and showed that it was significantly higher in the peripheral region compared to the central area. We attempted to control for this difference in the collection of the pathological twin samples by sampling the full thickness tissue at the placental periphery. However, it is possible that in a pathological state such as major growth restriction, INSL4 gene expression might vary in a different pattern and that the most significant aberrant area of its expression was missed. The identification of a mediator capable of inducing aberrant growth in the placenta is obviously important and further studies are needed to confirm our findings both in vivo and in vitro. In addition, the precise endogenous structure of this hormone needs to be known and its receptor identified before more firm conclusions can be drawn.
Acknowledgments
We thank the nurses and staff of the labor and delivery ward of Kapiolani Medical Center for Women and Children for their help with the tissue collection for this study. We also thank Dr. Lynn Iwamoto for help with the cell cycle study and Tercia Ku for the technical assistance with the flow cytometry.
Footnotes
Supported by NIH grant HD-24314 (GBG) and by grants to the University of Hawaii and Kapi’olani Medical Center under the Research Centers in Minority Institutions Program of the National Institutes of Health (RR1A1-03061 and RR-11091).
References
- 1.Ong K, Kratzsch J, Kiess W, Alspac study team. Costello M, Scott C, Dunger D. Size at birth and cord blood levels of insulin, insulin-like growth factor I (IGF-I), IGF-II, IGF-binding protein-I (IGFBP-I), IGFBP3, and the soluble IGF-II/mannose-6-phosphate receptor in term human infants. J Clin Endocrinol Metab. 2000;85:4266–4269. doi: 10.1210/jcem.85.11.6998. [DOI] [PubMed] [Google Scholar]
- 2.Giudice LC, deZegher F, Gargosky SE, Dsupin BA, De Las Fuentas L, Crystal RA, et al. Insulin-like growth factors and their binding proteins in the term and preterm human fetus and neonate with normal and extremes of intrauterine growth. J Clin Endocrinol Metab. 1995;80:1548–1555. doi: 10.1210/jcem.80.5.7538146. [DOI] [PubMed] [Google Scholar]
- 3.McIntyre HD, Serek R, Crane DI, Veveris-Lowe T, Parry A, Johnson S, Leung KC, Ho K, Bougoussa M, Hennen G, Igout A, Chan F-Y, Cowley D, Cotterill A, Barnard R. Placental growth hormone (GH), GH-binding protein, and insulin-like growth factor axis in normal, growth-retarded, and diabetic pregnancies: correlations with fetal growth. J Clin Endocrinol Metab. 2000;85:1143–1150. doi: 10.1210/jcem.85.3.6480. [DOI] [PubMed] [Google Scholar]
- 4.DeChiara TM, Efstratiadis A, Robertson EJ. A growth-deficiency phenotype in heterozygous mice carrying an insulin-like growth factor II gene disrupted by targeting. Nature. 1990;345:78–80. doi: 10.1038/345078a0. [DOI] [PubMed] [Google Scholar]
- 5.Millar LK, Reiny R, Yamamoto SY, Okazaki K, Webster L, Bryant-Greenwood GD. Relaxin causes proliferation of human amniotic epithelium by stimulation of insulin-like growth factor-II. Am J Obstet Gynecol. 2003;188:234–241. doi: 10.1067/mob.2003.80. [DOI] [PubMed] [Google Scholar]
- 6.Chassin D, Laurent A, Janneau JL, Berger R, Bellet D. Cloning of a new member of the insulin gene superfamily (INSL4) expressed in human placenta. Genomics. 1995;29:465–470. doi: 10.1006/geno.1995.9980. [DOI] [PubMed] [Google Scholar]
- 7.Lin F, Otvos L, Kumagai J, Tregear GW, Bathgate RAD, Wade JD. Synthetic human insulin 4 does not activate the G-protein-coupled receptors LGR7 or LGR8. J Pept Sci. 2004;10:257–264. doi: 10.1002/psc.521. [DOI] [PubMed] [Google Scholar]
- 8.Koman A, Cazaubon S, Couraud P, Ullrich A, Strosberg D. Molecular characterization and in vitro biological activity of Placentin, a new member of the insulin gene family. J Biol Chem. 1996;271:20238–20241. doi: 10.1074/jbc.271.34.20238. [DOI] [PubMed] [Google Scholar]
- 9.Laurent A, Rouillac C, Delezoide AL, Giovangrandi Y, Vekemans M, Bellet D, et al. Insulin-like 4 (INSL4) gene expression in human embryonic and trophoblast tissues. Mol Reprod Dev. 1998;51:123–129. doi: 10.1002/(SICI)1098-2795(199810)51:2<123::AID-MRD1>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 10.Veitia R, Laurent A, Quintana-Murci L, Ottolenghi C, Fellous M, Vidaud M, McElreavey K. The INSL4 gene maps close to WI-5527 at 9p24.1 → p23.3 clustered with two relaxin genes and outside the critical region for the monsomy 9p syndrome. Cytogenet Cell Genet. 1998;81:275–277. doi: 10.1159/000015045. [DOI] [PubMed] [Google Scholar]
- 11.Bieche I, Laurent A, Laurendeau I, Duret L, Giovangrandi Y, Frendo J-L, Olivi M, Fausser J-L, Evain-Brion D, Vidaud M. Placenta-specific INSL4 expression is mediated by a human endogenous retrovirus element. Biol Reprod. 2003;68:1422–1429. doi: 10.1095/biolreprod.102.010322. [DOI] [PubMed] [Google Scholar]
- 12.Mock P, Frydman R, Bellet D, Diawara DA, Lavaissiere L, Troalen F, Troalen F. Pro-EPIL forms are present in amniotic fluid and maternal serum during normal pregnancy. J Clin Endocrinol Metab. 1999;84:2253–2256. doi: 10.1210/jcem.84.6.5888. [DOI] [PubMed] [Google Scholar]
- 13.Mock P, Frydman R, Bellet D, Chassin D, Bischof P, Campana A, Bidart J-M. Expression of Pro-EPIL peptides encoded by the insulin-like 4 (INSL4) gene in chromosomally abnormal pregnancies. J Clin Endocrinol Metab. 2000;85:3941–3944. doi: 10.1210/jcem.85.10.6925. [DOI] [PubMed] [Google Scholar]
- 14.Büllesbach EE, Schwabe C. Synthesis and conformational analysis of the insulin-like 4 gene product. J Pept Res. 2001;57:77–83. doi: 10.1034/j.1399-3011.2001.00832.x. [DOI] [PubMed] [Google Scholar]
- 15.Goldenberg RL. The management of preterm labor. Obstet Gynecol. 2002;100:1020–1037. doi: 10.1016/s0029-7844(02)02212-3. [DOI] [PubMed] [Google Scholar]
- 16.Lackman F, Capewell V, Richardson B, daSilva O, Gagnon R. The risks of spontaneous preterm delivery and perinatal mortality in relation to size at birth according to fetal versus neonatal growth standards. Am J Obstet Gynecol. 2001;184:946–953. doi: 10.1067/mob.2001.111719. [DOI] [PubMed] [Google Scholar]
- 17.Bukowski R, Gahn D, Denning J, Saade G. Impairment of growth in fetuses destined to deliver preterm. Am J Obstet Gynecol. 2001;185:463–467. doi: 10.1067/mob.2001.115865. [DOI] [PubMed] [Google Scholar]
- 18.Keelen JA, Sato T, Mitchell MD. Interleukin (IL)-6 and IL-8 production by human amnion: regulation by cytokines, growth factors, glucocorticoids, phorbol esters, and bacterial lipopolysaccharide. Biol Reprod. 1997;57:1438–1444. doi: 10.1095/biolreprod57.6.1438. [DOI] [PubMed] [Google Scholar]
- 19.Casey ML, MacDonald PC. Interstitial collagen synthesis and processing in human amnion: a property of the mesenchymal cells. Biol Reprod. 1996;55:1253–1260. doi: 10.1095/biolreprod55.6.1253. [DOI] [PubMed] [Google Scholar]
- 20.Zong ZP, Fujikawa-Yamamoto K, Yamaguchi N, Chang YG, Murakami M, Odashima S, Ishikawa Y. Both low and high concentrations of staurosporine induce G1 arrest through down-regulation of cyclin E and cdk2 expression. Cell Struct Funct. 1999;24:457–463. doi: 10.1247/csf.24.457. [DOI] [PubMed] [Google Scholar]
- 21.Salafia CM, Vintzileos AM. Why all placentas should be examined by a pathologist in 1990. Am J Obstet Gynecol. 1990;163:1282–1293. doi: 10.1016/0002-9378(90)90708-f. [DOI] [PubMed] [Google Scholar]
- 22.Brandt B, Roetger A, Bidart JM, Packeisen J, Schier K, Mikesch JH, Kemming D, Boecker W, Yu D, Buerger H. Early placenta insulin-like growth factor (pro-EPIL) is overexpressed and secreted by c-erbB-2 positive cells with high invasion potential. Cancer Res. 2002;62:1020–1024. [PubMed] [Google Scholar]
- 23.Smith SC, Baker PN, Symonds EM. Placental apoptosis in normal human pregnancy. Am J Obstet Gynecol. 1997;177:57–65. doi: 10.1016/s0002-9378(97)70438-1. [DOI] [PubMed] [Google Scholar]
- 24.Almog B, Fainaru O, Gamzu R, Kupferminc MJ, Sasson R, Gold R, Lessing JB, Amsterdam A, Many A. Placental apoptosis in discordant twins. Placenta. 2002;23:331–336. doi: 10.1053/plac.2002.0788. [DOI] [PubMed] [Google Scholar]
- 25.Pidoux G, Gerbaud P, Laurendeau I, Guibourdenche J, Bertin G, Vidaud M, Evain-Brion D, Frendo J-L. Large variability of trophoblast gene expression within and between human normal term placentae. Placenta. 2004;25:469–47. doi: 10.1016/j.placenta.2003.10.016. [DOI] [PubMed] [Google Scholar]


