Abstract
Mesenteric branch arteries isolated from cannabinoid type 1 receptor knockout (CB1−/−) mice, their wild-type littermates (CB1+/+ mice), and C57BL/J wild-type mice were studied to test the hypothesis that murine arteries undergo high sensitivity Ca2+-induced relaxation that is CB1 receptor dependent. Confocal microscope analysis of mesenteric branch arteries from wild-type mice showed the presence of Ca2+ receptor–positive periadventitial nerves. Arterial segments of C57 control mice mounted on wire myographs contracted in response to 5 μmol/L norepinephrine and responded to the cumulative addition of extracellular Ca2+ with a concentration-dependent relaxation that reached a maximum of 72.0±6.3% of the prerelaxation tone and had an EC50 for Ca2+ of 2.90±0.54 mmol/L. The relaxation was antagonized by precontraction in buffer containing 100 mmol/L K+ and by pretreatment with 10 mmol/L tetraethylammonium. Arteries from CB1−/− and CB1+/+ mice also relaxed in response to extracellular Ca2+ with no differences being detected between the knockout and their littermate controls. SR141716A, a selective CB1 antagonist, caused concentration-dependent inhibition of Ca2+-induced relaxation in both the knockout and wild-type strains (60% inhibition at 1 μmol/L). O-1918, a cannabidiol analog, had a similar blocking effect in arteries of both wild-type and CB1−/− mice at 10 μmol/L. In contrast, 1 μmol/L SR144538, a cannabinoid type 2 receptor antagonist, or 50 μmol/L 18α-glycyrrhetinic acid, a gap junction blocker, were without effect. SR141716A (1 to 30 μmol/L) was also assessed for nonspecific actions on whole-cell K+ currents in isolated vascular smooth muscle cells. SR141716A inhibited macroscopic K+ currents at concentrations higher than those required to inhibit Ca2+-induced relaxation, and appeared to have little effect on currents through large conductance Ca2+-activated K+ channels. These data indicate that arteries of the mouse relax in response to cumulative addition of extracellular Ca2+ in a hyperpolarization-dependent manner and rule out a role for CB1 or CB2 receptors in this effect. The possible role of a nonclassical cannabinoid receptor is discussed.
Keywords: mesenteric arteries, calcium, relaxation, potassium channels
Over the past several years, a novel, extracellular Ca2+-activated, vasodilator pathway has been described in isolated rat arteries. The current model describing this system holds that elevation of extracellular Ca2+, as occurs in the interstitial compartments of some tissues, activates a Ca2+-sensing receptor localized on perivascular sensory nerves. Activation of this receptor causes the release of a transmitter that diffuses to underlying vascular smooth muscle and induces hyperpolarization-mediated relaxation through activation of a vascular smooth muscle cannabinoid type 1 (CB1) receptor.1 Among the findings that support this pathway are the demonstration that dorsal root ganglia and perivascular sensory nerves express a protein that is immunoreactive with antibodies raised against the parathyroid Ca2+ receptor2 and that the magnitude of Ca2+-induced relaxation positively correlates with Ca2+ receptor–positive nerve density.3 The relaxation is inhibited by nonselective antagonism of potassium (K+) channel activity with precontraction in depolarizing concentrations of KCl and pretreatment with tetraethyammonium2 and by selective blockade of large conductance KCa channels with iberiotoxin4 and charybdotoxin.5 Additional work has shown that the relaxation is blocked by the selective CB1 receptor antagonist SR141716A and is mimicked by anandamide,4 an endocannabinoid, which has been shown to be formed in neuronal tissue.6,7
Over the past 2 years, several reports have appeared that indicate that in addition to its high potency as a CB1 receptor antagonist,8 at concentrations of ≥10 μmol/L, SR141716A also has activity as an antagonist of type 2 cannabinoid (CB2) receptors,8 Ca2+ entry channels,9 KCa channels,9 and gap junctions.10 Although we have found that lower concentrations of SR141716A inhibit the mesenteric vasodilator response to anandamide,4 we believed it was important to obtain additional information regarding whether Ca2+-induced relaxation is mediated by the CB1 receptor. The CB1−/− mouse11 offers a direct means of testing the hypothesis that Ca2+-induced relaxation is mediated by a transmitter with activity at the CB1 receptor. We have therefore performed experiments to (1) determine whether arteries isolated from mice undergo extracellular Ca2+-induced relaxation and whether the relaxation shares properties with the relaxation observed in rats and (2) to test the hypothesis that Ca2+-induced relaxation is mediated by the CB1 receptor.
Methods
Animals
All experiments using animals were approved by the appropriate institutional animal care and use committees. CB1 receptor knockout (CB1−/−) mice and homozygous littermate controls (CB1+/+) were bred from founders that were generously provided by Dr Andreas Zimmer (Rheinische Friedrich-Wilhelms-Universitat Klinik und Poliklinik fur Psychiatrie und Psychotherapie Abteilung Molekulare Neurobiologie, Bonn, Germany) and that have been described in detail.11 Some control experiments were performed using tissue obtained from nonlittermate inbred C57BL/6 mice (Harlan Sprague Dawley, Indianapolis, Ind). Male and female animals were studied, and mesenteric tissue was isolated after the animals were killed by cervical dislocation. Male golden Syrian hamsters (9 to 12 weeks of age) were obtained from Charles Rivers Laboratories (Wilmington, Mass) and on the day of the experiment were killed by CO2 asphyxiation followed by cervical dislocation. Cremaster muscles were removed, and second- and third-order arterioles were isolated by hand dissection as described previously.12
Immunocytochemistry
Mesenteric branch arteries were microdissected from isolated mesenteries and prepared for immunostaining as described previously.2 Fixed and blocked segments were incubated overnight with a polyclonal anti-Ca2+ receptor antibody (1:50, Affinity Bioreagents) in the presence or absence of excess antigen. Anti-rabbit IgG conjugated with Texas Red (1:300, Molecular Probes) was used as the secondary antibody. Vessel segments were viewed using a Zeiss LSM 510 confocal microscope and 63× water immersion objective (Zeiss Instruments).
Biophysical Measurements
Isometric force generation was measured using previously described methods.2 Relaxation to Ca2+ or acetylcholine was assessed by cumulatively adding the agent to vessels that had been precontracted to a steady-state tension using 5 μmol/L norepinephrine (NE). The percentage of relaxation was calculated taking the magnitude of the prerelaxation tone as 100% of the amount that the vessel could relax. When the effect of an antagonist, ie, SR141716A, was assessed on the relaxation response, the vessel was pretreated with the compound for 10 to 15 minutes. Afterward, the contraction was induced by the addition of NE, and the response to the dilator assessed.
An expanded Biophysical Measurements section can be found in an online data supplement available at http://www.hypertensionaha.org.
Electrophysiology
Vascular smooth muscle cells were enzymatically isolated from hamster cremasteric arterioles using a papain digestion medium as described previously.12 Macroscopic K+ currents were measured using the conventional whole cell recording method.13 Currents were measured, and membrane potential was clamped with a Warner PC-505A patch-clamp amplifier (Warner Instrument Corp), filtered at 1 kHz, and sampled at 5 kHz. As reported previously, all currents were normalized to cell capacitance to account for any differences in cell size. Cells were held at −60 mV and then stepped, for 400 ms, to test potentials from −90 to 60 mV in 10-mV increments. The average current during the last 200 ms of the test pulse was then measured at each potential and used to construct current/voltage relationships.
An expanded Electrophysiology section can be found in an online data supplement available at http://www.hypertensionaha.org.
Materials
SR141716A and SR144538 were obtained from Sanofi Recherche and dissolved in absolute ethanol; O-1918 was synthesized by Dr Raj Razdan (Organix Inc, Woburn, Mass) and dissolved in ethanol. All other chemicals were of reagent grade or better and obtained from Sigma Chemical or other commercial suppliers, as dictated by the purchasing regulations of the University of North Carolina System.
Statistical Analysis
The agonist concentration eliciting 50% of the maximal relaxation (EC50) was determined from plots of the percentage of initial tension versus the concentration of agonist. All data are presented as mean±SEM, and statistical analysis was performed using the SYSTAT software package. Comparisons among groups were performed using ANOVA with a repeated measures design when appropriate. A value of P<0.05 was taken to indicate a statistically significant difference.
Results
Whole-mount sections of mouse mesenteric branch arteries were stained with anti-Ca2+ receptor antibody and viewed using a laser confocal microscope (Figure 1). Analysis of sections from at least 6 mice showed that nerve fibers located in the adventitia stained positively for Ca2+ receptor protein, and Ca2+ receptor–positive staining was not present elsewhere in the vessel wall (Figure 1A and 1B). Moreover, preincubation of the primary antibody with blocking peptide or staining with secondary antibody alone eliminated nerve staining (Figure 1C).
Figure 1.
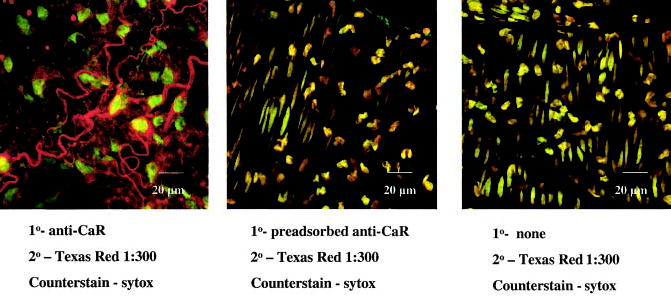
3D confocal images of mesenteric branch arteries of wild-type C57BL/6 mice. Left, Ca2+ receptor–positive nerves in the adventitial layer and nuclei of adjacent cells. Middle, Reduction in nerve staining after preadsorption of primary antibody with excess antigen. Right, Absence of staining when primary antibody is omitted from the assay. Note that middle and right panels contain oblong nuclei from smooth muscle cells in the tunica media. Photos were taken with a 63× water immersion objective.
Mesenteric branch arteries isolated from C57 control mice and precontracted with 5 μmol/L NE relaxed in response to cumulative addition of extracellular Ca2+, with a maximal relaxation response of 72.0±6.3% of the prerelaxation tone (n=6) and an EC50 for Ca2+ of 2.9±0.54 mmol/L (Figure 2A). To learn whether Ca2+-induced relaxation of mouse arteries is dependent on functional K+ channels, the relaxation response was also determined in vessels precontracted with 100 mmol/L K+ or pretreated with 10 mmol/L tetraethylammonium (TEA). Precontraction with a depolarizing concentration of K+ converted the Ca2+-induced relaxation into a Ca2+-induced contraction (Figure 2A). Moreover, pretreatment with 10 mmol/L TEA significantly inhibited the relaxation induced by cumulative addition of extracellular Ca2+ (Figure 2B). Because a residual relaxation to Ca2+ was observed in the presence of 10 mmol/L TEA, we tested the hypothesis that this component is mediated by NO. Pretreatment with 0.3 mmol/L NG-nitro-l-arginine methyl ester, however, was without further inhibitory effect (Figure 2B).
Figure 2.
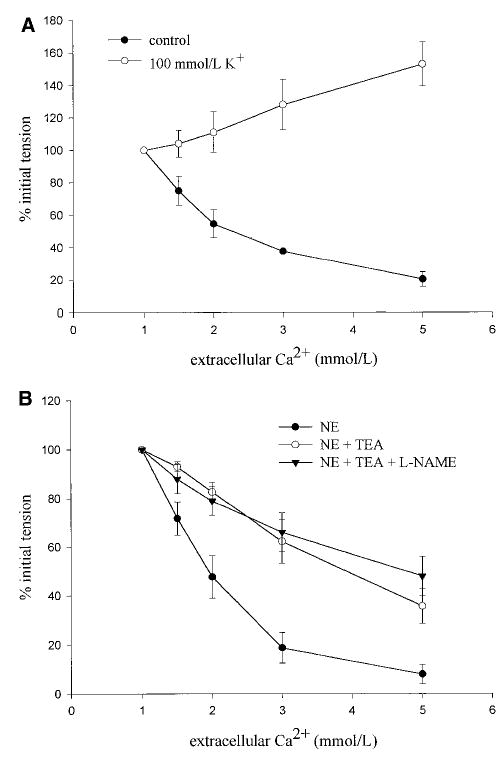
Response of mesenteric branch arteries of wild-type C57BL/6 mice to the cumulative addition of extracellular Ca2+ after precontraction with 5 μmol/L NE or 100 mmol/L K+ (A) or after precontraction with 5 μmol/L NE and pretreatment with 10 mmol/L TEA or a mixture of 10 mmol/L TEA and 0.3 mmol/L NG-nitro-l-arginine methyl ester (L-NAME, B). Values are mean±SEM, *P<0.05 vs NE control, n=3 to 5 per group.
Branch arteries isolated from CB1−/− animals and CB1+/+ littermates were also studied. Ca2+ caused a concentration-dependent relaxation of CB1−/− arteries that was not different from that observed in vessels isolated from CB1+/+ control animals (maximal relaxation response, 75.5±6.9%, P=0.954; EC50, 3.2±0.28 mmol/L, n=6, P=0.714) (Figure 3A). The response of CB1+/+ and CB1−/− arteries to the cumulative addition of acetylcholine was also assessed. Acetylcholine induced concentration-dependent relaxation of arteries isolated from both the wild-type and knockout strains (data not shown). No differences in either the maximal response or the sensitivity of the arteries to acetylcholine were detected (maximal CB1+/+ response versus CB1−/−, 76.9±7.5% versus 78.3±10.7%, n=3 and n=4, respectively, P=0.998; EC50 CB1+/+ versus CB1−/−, 0.71±0.42 versus 0.21±0.15 μmol/L, P=0.888).
Figure 3.
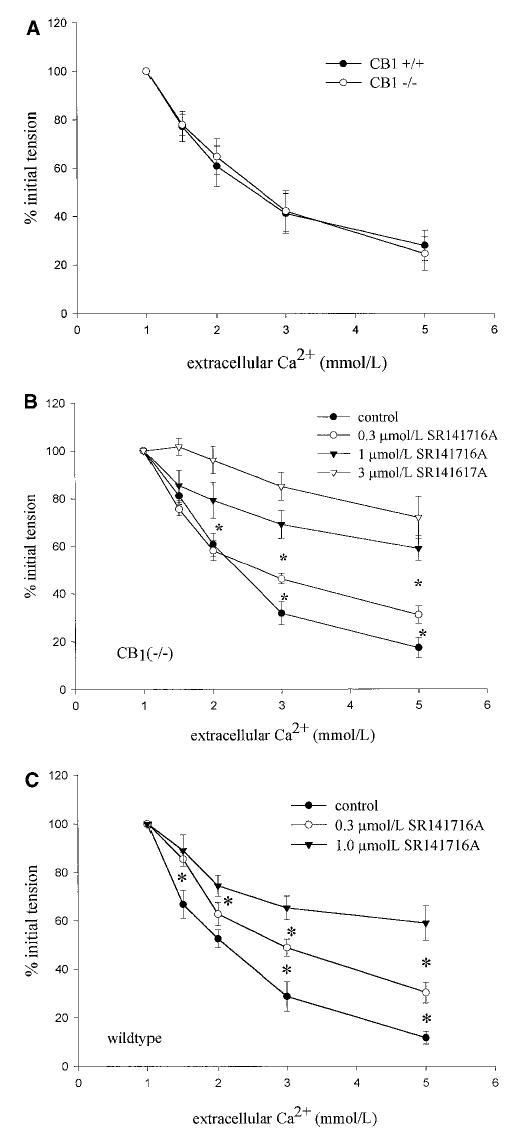
A, Response of mesenteric branch arteries isolated from CB1+/+ and CB1−/− mice to the cumulative addition of extracellular Ca2+ after precontraction with 5 μmol/L NE. Values are mean± SEM, n=6; no significant differences were detected. Effect of 0.3 and 1.0 μmol/L SR141716A on Ca2+-induced relaxation of mesenteric branch arteries isolated from CB1−/− mice (B) and wild-type control mice (C). Values are mean± SEM, n=4 to 6. *P<0.05 between points.
Preincubation of vessels with increasing concentrations of the CB1 antagonist SR141716A dose-dependently inhibited Ca2+-induced relaxation of NE-precontracted arteries isolated from both the CB1−/− strain (Figure 3B) and the CB1+/+ control (Figure 3C). These data indicate that the threshold for SR141617A inhibition of Ca2+-induced relaxation is <300 nmol/L and that the IC50 lies between 300 nmol/L and 1 μmol/L.
To learn whether the blockade by SR141716A might be mediated by antagonist activity at the CB2 receptor, we assessed the effect of pretreatment with the selective CB2 antagonist SR144538.14 Pretreatment with 1 μmol/L SR144538 had no effect on relaxation induced by Ca2+ in arteries isolated from either the knockout or wild-type animals (Figure 4A). To assess the possible role of gap junctional activity in the relaxation response, the effect of preincubation with 18α-glycyrrhetinic acid (50 μmol/L), a gap junction blocker10 was assessed. 18α-glycyrrhetinic acid had no effect on the relaxation response determined in vessels from 4 animals (Figure 4B). Our previous data indicate that Ca2+-induced relaxation is inhibited by iberiotoxin, a selective inhibitor of large conduct KCa channels.4 To learn whether a possible nonselective effect of SR141716A at K+ channels plays a role in the inhibition of Ca2+-induced relaxation, we assessed the effect of a range of concentrations of SR141716A on whole-cell K+ channel currents in isolated vascular smooth muscle cells. SR141716A inhibited macroscopic K+ currents in a concentration-dependent fashion, (Figure 5A), with maximal effects observed at 10 μmol/L (Figure 5B). To learn whether SR141716A blocks KCa channel activity, we tested whether the inhibitory action of the antagonist was additive with respect to TEA. Initial experiments showed that TEA (1 mmol/L) and iberiotoxin (100 nmol/L) inhibit whole-cell K+ channel currents to a similar degree and in a nonadditive fashion (Figure 6A). In contrast, the K+-blocking effect of SR141716A was clearly additive to the inhibition produced by 1 mmol/L TEA (Figure 6B and 6C) or 100 nmol/L iberiotoxin (n=3, data not shown). These observations suggest that SR141716A has little effect on currents through large conductance KCa channels.
Figure 4.
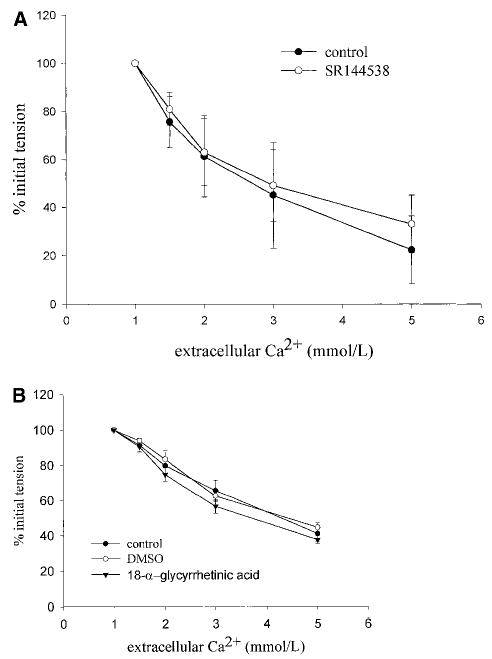
Effect of pretreatment with 3 μmol/L SR144538 on Ca2+-induced relaxation of arteries isolated from CB1−/− mice (A) or effect of preincubation of the artery with 50 μmol/L 18α-glycyrrhetinic acid on arteries isolated from wild-type C57BL/6 mice (B). Values are mean±SEM, n=3 to 4. No effect of either antagonist was detected.
Figure 5.
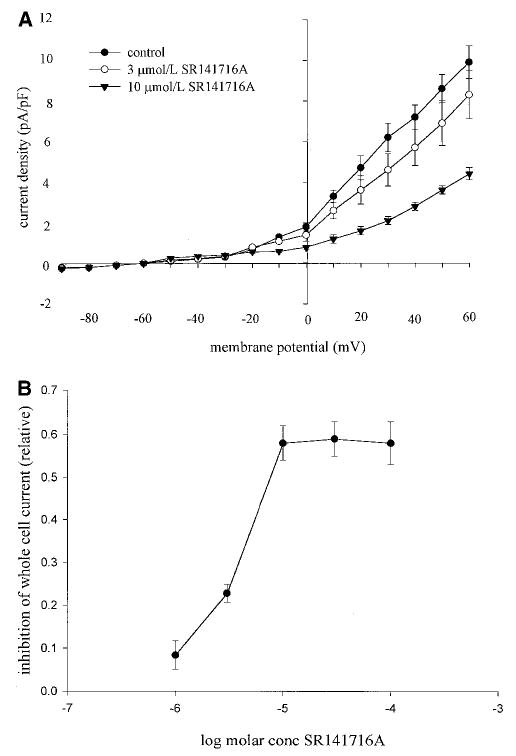
A, Effect of SR141716A on whole-cell K+ currents in isolated vascular smooth muscle cells under control conditions or in the presence of 3 and 10 μmol/L SR141716A. Values are mean current densities±SEM, n=5 to 6. Both concentrations significantly inhibited currents (P<0.05). B, Concentration response data for the inhibitory effect of SR141716A on whole-cell K+ currents. Values are mean inhibition±SEM, n=3 to 13, measured by stepping to 60 mV from a holding potential of −60 mV. SR141716A inhibited the currents in a concentration-dependent fashion between 1 and 10 μmol/L.
Figure 6.
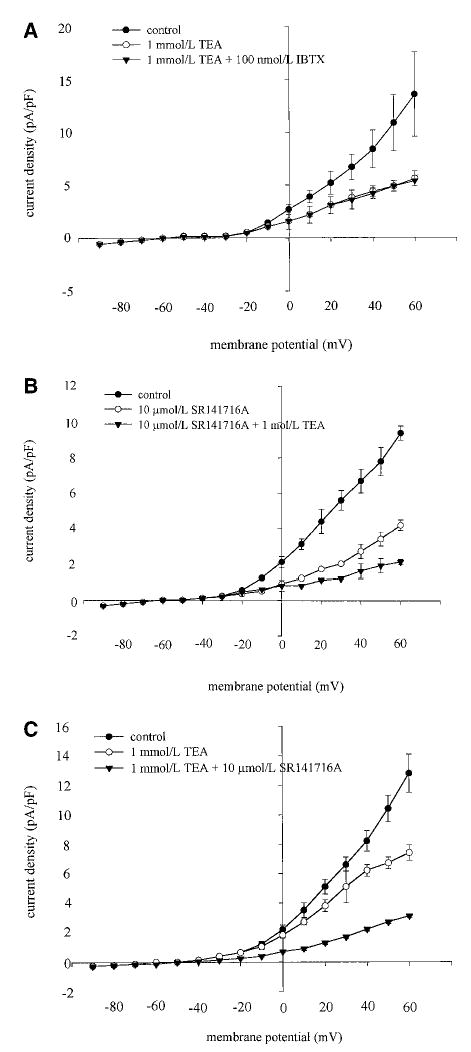
Inhibition of whole-cell K+ currents by SR141716A is additive with TEA. A, Values are mean current density±SEM (n=5) in the absence (control) or presence of either 1 mmol/L TEA or 1 mmol/L TEA and 100 nmol/L iberiotoxin. Note that iberiotoxin produced no additional inhibition to that produced by TEA, indicating that TEA was maximally blocking currents through large conductance KCa channels. B, Values are mean current densities±SEM (n=6) in the absence (control) or presence of either 10 μmol/L SR141716A or 10 μmol/L SR141716A and 1 mmol/L TEA. TEA caused additional inhibition of currents above that produced by SR141716A. C, Values are mean current densities±SEM (n=5) in the absence (control) or presence of either 1 mmol/L TEA or 1 mmol/L TEA and 10 μmol/L SR141716A. SR141716A produced additional inhibition in addition to that produced by TEA, indicating that SR141716A is inhibiting currents through channels other than large-conductance KCa channels.
A previous report15 from one of our laboratories (G.K.) showed that abnormal-cannabidiol induces relaxation of the isolated perfused mesenteric artery bed of the rat and mouse and that the relaxation is blocked by cannabidiol and by SR141716A. We therefore assessed the effect of a novel cannabidiol analog, O-1918, that has been found to block abnormal cannabidiol-induced relaxation in isolated rat arteries (S. Batkai and G. Kunos, 2001, unpublished observations). Pretreatment of arteries isolated from C57BL/6 wild-type mice and CB1−/− mice with 10 μmol/L O-1918 for 15 minutes caused significant inhibition of Ca2+-induced relaxation (Figure 7A and 7B), whereas it was without effect on relaxation induced by acetylcholine (Figure 7C).
Figure 7.
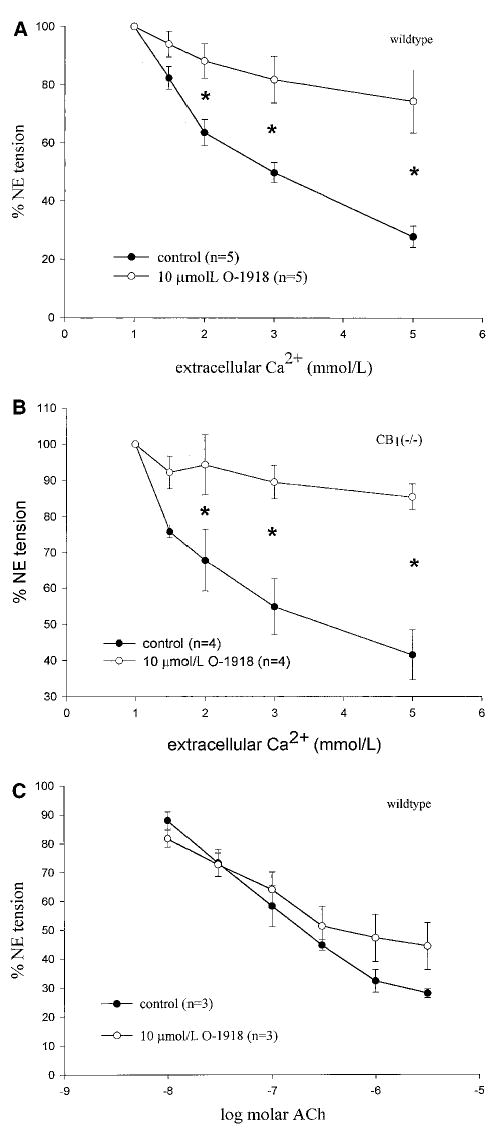
Effect of pretreatment with 10 μmol/L of the cannabidiol analog O-1918 on Ca2+-induced relaxation of arteries isolated from C57BL/6 mice (A) and CB1−/− mice (B). Values are mean±SEM, n=4 or 5 per group, *P <0.05. C, Acetylcholine-induced relaxation in arteries of C57BL/6 mice. Values are mean±SEM, n=3 per group. No differences were detected.
Discussion
The present studies were performed to determine whether arteries isolated from mice undergo extracellular Ca2+-induced relaxation, to learn whether the relaxation shares properties with Ca2+-induced relaxation observed in rats, and to test the hypothesis that Ca2+-induced relaxation is mediated by the CB1 receptor. The major new findings are that (1) murine mesenteric branch arteries are supplied with Ca2+ receptor–positive periadventitial nerves and relax in response to the cumulative addition of extracellular Ca2+; (2) the relaxation is blocked by conditions that inhibit K+ channel–dependent relaxation; (3) the relaxation occurs in the absence of CB1 receptors; and (4) the relaxation is antagonized by SR141716A in arteries of the CB1 receptor knockout mouse.
Earlier studies, performed using arteries isolated from the rat, showed that when care is taken to preserve the integrity of the vascular adventitia, cumulative addition of extracellular Ca2+ causes concentration-dependent relaxation.2 In recent studies of arterial segments mounted on 14-μm gold wires, we have found EC50 values for Ca2+ of 1.45±0.02 mmol/L (R.D. Bukoski, 2001, unpublished observations), which is well within the range of the concentrations that have been shown to occur in the interstitial fluid compartment of the small bowel16 and renal cortex.17 The relaxation is attenuated by sensory denervation and correlates with the presence of Ca2+ receptor–positive nerve fibers.2 Ca2+-induced relaxation of rat arteries has also been shown to be inhibited by blockers of the large conductance KCa channel4,5 and by SR141716A4, a compound with CB1 antagonist activity.8 Moreover, the relaxation is mimicked by anandamide, an endocannabinoid CB1 receptor agonist18 with hyperpolarizing vasodilator activity.19–21 Together, these observations have led to the hypothesis that changes in the concentration of Ca2+ in the interstitial fluid compartment are sensed by a sensory nerve Ca2+ receptor, and activation of this receptor is coupled with the release of an endocannabinoid-like hyperpolarizing vasodilator transmitter with activity at the CB1 receptor.1
The present studies demonstrate that perivascular nerves of mouse mesenteric branch arteries express Ca2+ receptor protein. These findings extend our prior demonstration that dorsal root ganglia of the rat express cDNA encoding a protein that is homologous with rat kidney Ca2+ receptor2; that DRG and mesenteric arteries express a protein that is immunoreactive with the parathyroid Ca2+ receptor2; and that arteries isolated from the mesentery, kidney, and brain of the rat express Ca2+ receptor–positive protein.22 As in the rat, Ca2+-induced relaxation of the mouse mesenteric artery is completely blocked by precontraction of the vessel segment with a depolarizing concentration of potassium chloride and is significantly attenuated by blockade of K+ channel activity with 10 mmol/L TEA.2 These observations suggest the involvement of K+ channels and a hyperpolarization-dependent mechanism of relaxation. To our knowledge, this is the first demonstration of Ca2+ receptor expression in perivascular nerves and high sensitivity Ca2+-induced relaxation of a species other than the rat.
The second new finding of the present study is the observation that Ca2+-induced relaxation occurs in mesenteric branch artery segments isolated from CB1 receptor knockout mice. These experiments were designed to follow up on the prior observation that Ca2+-induced relaxation in the rat is blocked by SR141716A and to directly test the hypothesis that Ca2+-induced relaxation requires an active CB1 receptor. The results clearly indicate that the CB1 receptor is not involved in the Ca2+-induced relaxation event in the mouse.
A third finding that merits discussion is our observation that Ca2+-induced relaxation of mouse artery is inhibited by SR141716A. This supports our earlier finding obtained using the rat.4 In addition, the observation that the sensitivity of Ca2+-induced relaxation to inhibition by SR141716A is similar in arteries isolated from the CB1−/− knockout and wild-type control argues against the possibility that SR141716A inhibits Ca2+-induced relaxation via CB1 receptors in wild-type mice, but suggests that a different site is involved in the inhibition observed in CB1 receptor knockout mice.
As noted in the Introduction, one possible explanation for our finding that Ca2+-induced relaxation is intact in the CB1 knockout mouse, yet is blocked by SR141716A, is that the blockade by SR141716A is mediated by nonspecific actions of the compound. As noted above, it has been reported that at high micromolar concentrations, SR141716A blocks CB2 receptors,8 gap junctions,10 Ca2+ entry channels,10 and KCa channels.10 Our finding that SR144538, a selective CB2 antagonist, had no effect on Ca2+-induced relaxation in the CB1 knockout animal indicates that SR141716A is not blocking the relaxation through a CB2 receptor effect. Moreover, our finding that 18α-glycyrrhetinic acid, a compound with gap junction–blocking activity,10 has no effect on Ca2+ relaxation in the rat makes it unlikely that SR141716A is attenuating Ca2+-induced relaxation through a gap junction–blocking effect. It also seems unlikely that SR141716A is blocking Ca2+-induced relaxation by inhibiting L-type Ca2+ channels because L-type Ca2+ channel inhibition is generally associated with a decrease in agonist-induced contraction and not blockade of a relaxation event.
In view of the fact that Ca2+-induced relaxation is antagonized by the selective large conductance KCa channel antagonist iberiotoxin,4 we were particularly concerned about the contribution of possible K+ channel–blocking activity of SR1416171A, as has been reported by White and Hiley.9 To directly address this issue, we determined the effect of a range of concentrations of SR141716A on whole-cell K+ channel currents in isolated vascular smooth muscle cells. The results showed clearly that SR141716A causes concentration-dependent inhibition of these currents. Two findings are of particular interest for the present study. One is that 1 μmol/L SR141716A caused <8% inhibition of K+ channel current, whereas the same concentration inhibited Ca2+-induced relaxation by >60%. A second finding is that the effect of SR141716A is additive to the effect of TEA and iberiotoxin. This suggests that SR141716A inhibits K+ channels other than KCa channels, presumably voltage-dependent K+ channels, which are less sensitive to SR141716A than the site at which SR141716A inhibits Ca2+-induced relaxation. In view of these observations, it seems unlikely that the inhibitory effect of SR141716A on Ca2+-induced relaxation is mediated by an inhibition of either KCa or voltage-dependent K+ channel activity.
A second possible explanation for our finding that Ca2+-induced relaxation is intact in the CB1 knockout mouse, yet is blocked by SR141716, is that SR141716A is blocking a nonclassical cannabinoid receptor. Kunos and colleagues15 have recently demonstrated that abnormal cannabidiol, an analog of the nonpsychoactive marijuana-constituent cannabidiol,23 causes relaxation of the isolated rat mesenteric bed. They further found that both anandamide and abnormal cannabidiol have mesenteric vasodilator activity in the CB1 knockout mouse, and the effect of both compounds is antagonized by SR141716A or cannabidiol.15 These findings have been interpreted to indicate that anandamide and other endocannabinoids can modulate vascular function by activating a nonclassical cannabinoid receptor. Of interest, in the present report, we show that O-1918, a novel analog of cannabidiol that blocks abnormal cannabidiol-induced relaxation in isolated rat and mouse mesenteric arteries (S. Batkai and G. Kunos, 2001, unpublished observations), blocks Ca2+-induced but not acetylcholine relaxation in isolated mouse arteries. This observation provides further support for the hypothesis that Ca2+-induced relaxation is mediated by sensory nerve dependent release of an endocannabinoid, possibly anandamide, which diffuses to underlying vascular smooth muscle and activates a specific anandamide receptor, which induces hyperpolarizing relaxation.
Supplementary Material
Acknowledgments
We thank Dr Andreas Zimmer for providing the breeding pairs for the CB1 receptor knockout mice and their controls and Dr Raj Razdan for the synthesis of compound O-1918. The authors acknowledge the expert secretarial assistance of Barbara Manning. This work was supported by grants from the National Institutes of Health: HL 54901, HL59868, and HL64761 (R.D.B.) and HL32469 (W.F.J.).
References
- 1.Bukoski RD. Dietary Ca2+ and blood pressure: evidence that Ca2+ sensing receptor activated, sensory nerve dilator activity couples changes in interstitial Ca2+ with vascular tone. Nephrol Dialysis Transplant. 2001;16:218–221. doi: 10.1093/ndt/16.2.218. [DOI] [PubMed] [Google Scholar]
- 2.Bukoski RD, Bian K, Wang Y, Mupanomunda M. Perivascular sensory nerve Ca2+ receptor and Ca2+-induced relaxation of isolated arteries. Hypertension. 1997;30:1431–1439. doi: 10.1161/01.hyp.30.6.1431. [DOI] [PubMed] [Google Scholar]
- 3.Mupanomunda M, Wang Y, Bukoski RD. Effect of chronic sensory denervation on Ca2+-induced relaxation of isolated mesenteric resistance arteries. Am J Physiol. 1998;274:H1655–H1661. doi: 10.1152/ajpheart.1998.274.5.H1655. [DOI] [PubMed] [Google Scholar]
- 4.Ishioka N, Bukoski RD. A role for N-arachidonylethanolamine (anandamide) as the mediator of sensory nerve-dependent Ca2+-induced relaxation. J Pharmacol Exp Ther. 1999;289:245–250. [PubMed] [Google Scholar]
- 5.Bian K, Ishibashi K, Bukoski RD. Modulation of resistance artery force generation by extracellular Ca2+ Am J Physiol. 1995;269:H230–H238. doi: 10.1152/ajpheart.1995.269.1.H230. [DOI] [PubMed] [Google Scholar]
- 6.Devane WA, Hanus L, Breuer A, Pertwee RG, Stevenson LA, Griffin G, Gibson D, Mandelbaum A, Etinger A, Mechoulam R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science. 1992;258:1946–1949. doi: 10.1126/science.1470919. [DOI] [PubMed] [Google Scholar]
- 7.Devane WA, Axelrod J. Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc Natl Acad Sci U S A. 1994;91:6698–6701. doi: 10.1073/pnas.91.14.6698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, Martinez S, Maruani J, Neliat G, Caput D. SR 141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- 9.White R, Hiley CR. The actions of the cannabinoid receptor antagonist, SR 141716A, in the rat isolated mesenteric artery. Br J Pharmacol. 1998;125:689–696. doi: 10.1038/sj.bjp.0702127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chaytor AT, Martin PE, Evans WH, Randall MD, Griffith TM. The endothelial component of cannabinoid-induced relaxation in rabbit mesenteric artery depends on gap junctional communication. J Physiol (London) 1999;520(pt 2):539–550. doi: 10.1111/j.1469-7793.1999.00539.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zimmer A, Zimmer AM, Hohmann AG, Herkenham M, Bonner TI. Increased mortality, hypoactivity, and hypoalgesia in cannabinoid CB1 receptor knockout mice. Proc Natl Acad Sci U S A. 1999;96:5780–5785. doi: 10.1073/pnas.96.10.5780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jackson WF, Huebner JM, Rusch NJ. Enzymatic isolation and characterization of single vascular smooth muscle cells from cremasteric arterioles. Microcirculation. 1997;4:35–50. doi: 10.3109/10739689709148316. [DOI] [PubMed] [Google Scholar]
- 13.Hamill OP, Marty A, Neher B, Sakmann B, Sigworth FJ. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 1981;391:85–100. doi: 10.1007/BF00656997. [DOI] [PubMed] [Google Scholar]
- 14.Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, Oustric D, Sarran M, Bouaboula M, Calandra B, Portier M, Shire D, Breliere JC, Le Fur GL. SR144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- 15.Járai Z, Wagner JA, Varga K, Lake KD, Compton DR, Martin BR, Zimmer AM, Bonner TI, Buckley NE, Mezey E, Razdan RK, Zimmer A, Kunos G. Cannabinoid-induced mesenteric vasodilation through an endothelial site distinct from CB1 and CB2 receptors. Proc Natl Acad Sci U S A. 1999;96:14136–14141. doi: 10.1073/pnas.96.24.14136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mupanomunda MM, Ishioka N, Bukoski RD. Interstitial Ca2+ undergoes dynamic changes over a range that activates the perivascular sensory nerve Ca2+ receptor. Am J Physiol. 1999;276:H1035–H1042. doi: 10.1152/ajpheart.1999.276.3.H1035. [DOI] [PubMed] [Google Scholar]
- 17.Mupanomunda MM, Tian B, Ishioka N, Bukoski RD. Renal interstitial Ca2+ Am J Physiol. 2000;278:F644–F649. doi: 10.1152/ajprenal.2000.278.4.F644. [DOI] [PubMed] [Google Scholar]
- 18.Felder CC, Briley EM, Axelrod J, Simpson JT, Mackie K, Devane WA. Anandamide, an endogenous cannabimimetic eicosanoid, binds to the cloned human cannabinoid receptor and stimulates receptor-mediated signal transduction. Proc Natl Acad Sci U S A. 1993;90:7656–7660. doi: 10.1073/pnas.90.16.7656. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Randall MD, Alexander SPH, Bennett T, Boyd EA, Fry JR, Gardiner SH, Kemp PA, McCulloch AI, Kendall DA. An endogenous cannabinoid as an endothelium-derived vasorelaxant. Biochem Biophys Res Commun. 1996;229:114–120. doi: 10.1006/bbrc.1996.1766. [DOI] [PubMed] [Google Scholar]
- 20.Plane F, Holland M, Waldron GJ, Garland CJ, Boyle JP. Evidence that anandamide and EDHF act via different mechanisms in rat mesenteric arteries. Br J Pharmacol. 1997;121:1509–1511. doi: 10.1038/sj.bjp.0701361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White R, Hiley CR. A comparison of EDHF-mediated and anandamide-induced relaxations in the rat isolated mesenteric artery. Br J Pharmacol. 1997;122:1573–1584. doi: 10.1038/sj.bjp.0701546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang Y, Bukoski RD. Use of acute phenolic denervation to show the neuronal dependence of Ca2+-induced relaxation of isolated arteries. Life Sci. 1999;64:887–894. doi: 10.1016/s0024-3205(99)00009-0. [DOI] [PubMed] [Google Scholar]
- 23.Adams MD, Earnhardt JT, Martin BR, Harris LS, Dewey WL, Razdan RK. A cannabinoid with cardiovascular activity but no overt behavioral effects. Experientia. 1977;33:1204–1205. doi: 10.1007/BF01922330. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


