Abstract
Background
All of the available diagnostic tests for deep venous thrombosis (DVT) have limitations for excluding acute recurrent DVT. Measurement of plasma d-dimer by using an automated quantitative assay may be useful as a rapid exclusion test in patients with suspected recurrent DVT.
Objective
To test the safety of withholding additional diagnostic testing and heparin treatment in patients who have a negative d-dimer result at presentation (using the automated quantitative assay STA-Liatest d-di), regardless of their symptoms.
Design
Prospective cohort study.
Setting
Academic medical center in the United States.
Patients
300 consecutive patients with suspected recurrent DVT.
Intervention
Patients underwent d-dimer testing at presentation. In patients with negative d-dimer results, heparin therapy was withheld, and no further diagnostic testing for DVT was done as part of the initial evaluation. Patients with positive d-dimer results underwent compression ultrasonography.
Measurements
The primary outcome measure was a diagnosis of new symptomatic venous thromboembolism confirmed by diagnostic testing during the 3-month follow-up period.
Results
Of the 300 study patients, the d-dimer result was negative at presentation in 134 patients (45%; negative cohort) and positive at presentation in 166 patients. Of the 166 patients, compression ultrasonography documented new DVT in 54 patients. Compression ultrasonography findings were normal in 79 patients and were inconclusive in 33 patients. After 3 months of follow-up, 1 of 134 patients in the negative cohort had confirmed venous thromboembolism (0.75% [95% CI, 0.02% to 4.09%]). Venous thromboembolism on follow-up could not be definitively excluded in 5 patients with recurrent leg symptoms and in 1 patient who died. If these patients are considered to have venous thromboembolism, the incidence during the 3-month follow-up period would be 6.0% (CI, 2.6% to 11.4%) (8 of 134 patients).
Limitations
There is no accepted diagnostic reference standard for recurrent DVT. The precision of the estimate of the incidence of venous thromboembolism on follow-up and the generalizability to settings other than an academic health center should be evaluated.
Conclusions
Measurement of plasma d-dimer by using the automated quantitative assay STA-Liatest d-di seems to provide a simple method for excluding acute recurrent DVT in symptomatic patients.
Deep venous thrombosis is a common condition that affects more than 250 000 patients each year in the United States (1, 2). Symptoms of recurrent DVT, including leg pain or swelling, occur in one third of affected patients despite adequate anticoagulant treatment (3). Clinical diagnosis is inaccurate for distinguishing new, recurrent DVT from the post-thrombotic syndrome or other causes of leg pain and swelling. Diagnostic testing is needed because two thirds of patients with a clinical suspicion of recurrent DVT are shown to be free of acute thrombosis (4).
Acute recurrent DVT is confirmed by 1) the finding of a new noncompressible vein segment on compression ultrasonography, 2) a newly abnormal impedance plethysmography result, or 3) the finding of a new intraluminal filling defect on venography (3–5). However, all of the available diagnostic tests have limitations for excluding acute recurrent DVT. The results of compression ultrasonography may be persistently abnormal for 1 year in 50% of patients and longer in others (6–8). The combined use of impedance plethysmography and radiofibrinogen leg scanning has been useful for excluding recurrent DVT (4, 9). However, impedance plethysmography is no longer available, and I125 fibrinogen leg scanning is no longer used because of concern about potential viral transmission associated with fibrinogen injection. Venography also has limitations for excluding the diagnosis of recurrent DVT due to obliteration and recanalization of the previously affected venous segment or nonfilled venous segments (5). Nuclear venous imaging with technetium-labeled platelet glycoprotein IIb/IIIa receptor antagonists is a promising approach, but it has substantial limitations related to observer variation (10). Therefore, nuclear venous imaging requires additional evaluation before it is recommended.
Because of the limitations of the available diagnostic tests for DVT, there has been interest in the use of the laboratory assay for plasma d-dimer as an exclusion test. Automated rapid quantitative tests for detecting d-dimer in plasma have recently become available. Studies in patients with a suspected first episode of venous thromboembolism have shown that some of these assays have high sensitivity for acute DVT or pulmonary embolism (11). d-dimer has been used safely as an exclusion test in patients presenting with their first episode of DVT (12–14). The sensitivity of the STA-Liatest d-di (Diagnostica Stago, Asnieres-sur-Seine, France, and Parsippany, New Jersey) has been reported to be 96% to 100% in patients with suspected first-episode DVT or symptomatic pulmonary embolism (15, 16). However, the safety of using d-dimer as an exclusion test in patients with suspected recurrent DVT is uncertain. If d-dimer could be safely used to exclude acute recurrent DVT, an important unmet clinical need would be fulfilled.
Context
Acute recurrent deep venous thrombosis (DVT) is often difficult to differentiate from post-thrombotic syndrome, and each of the established diagnostic methods has limitations. A reliable test that excludes the diagnosis would be clinically useful.
Contribution
Patients with suspected recurrent DVT underwent d-dimer testing. Heparin was withheld or withdrawn in patients with negative results, and no additional testing was performed, regardless of symptoms. Three-month follow-up showed a low incidence (0.75% [95% CI, 0.02% to 4.09%]) of confirmed DVT in patients with a negative d-dimer result.
Implications
d-Dimer testing may obviate the need to perform additional testing in up to two thirds of patients being evaluated for recurrent DVT.
–The Editors
We performed a prospective cohort study of patients with clinically suspected recurrent DVT. The objective was to test the safety of withholding anticoagulant treatment and additional diagnostic testing in patients who have a negative d-dimer result at presentation. Because all of the standard diagnostic tests have limitations for the diagnosis of recurrent DVT, we defined criteria for the presence or absence of recurrent DVT a priori and then used long-term follow-up to test the validity of negative d-dimer results.
METHODS
Patients and Study Protocol
We conducted the study at the University of Oklahoma Health Sciences Center teaching hospitals, the Oklahoma University Medical Center, and the Veterans Affairs Medical Center. The Institutional Review Board of the University of Oklahoma Health Sciences Center approved the study.
We selected consecutive patients with a history of DVT confirmed by diagnostic testing. Patients were eligible for the study if they presented with symptoms and signs of recurrent DVT in either leg and if they were referred by their physician to the vascular clinic or the noninvasive vascular testing laboratory. The study sample included inpatients and outpatients.
Reasons for ineligibility were as follows: 1) signs and symptoms of upper-extremity DVT, 2) a history of pelvic venous thrombosis, 3) recent pelvic surgery, 4) an indwelling central line (upper extremity or femoral), 5) current pregnancy or delivery less than 1 month previously, 6) inability to undergo compression ultrasonography because of physical or technical limitations, or 7) inability to return for follow-up testing or visits. Patients receiving warfarin therapy or those receiving less than 24 hours of heparin or low-molecular-weight heparin therapy were eligible for the study.
All eligible patients who gave informed consent underwent d-dimer testing using the STA-Liatest d-di. The automated plasma d-dimer assay was performed by using an STA Compact Diagnostica Stago assay instrument (Diagnostica Stago). A negative d-dimer result was defined before the study as a plasma concentration of 0.47 μg/mL or less, as recommended by the manufacturer. All patients were then managed according to the study protocol (Figure). All patients with negative d-dimer test results had unfractionated heparin and low-molecular-weight heparin withheld or withdrawn, regardless of their symptoms. No additional diagnostic testing for DVT was performed as part of this initial evaluation for recurrent DVT in this group of patients (negative cohort).
Figure. Study protocol for patients with suspected recurrent deep venous thrombosis (DVT).
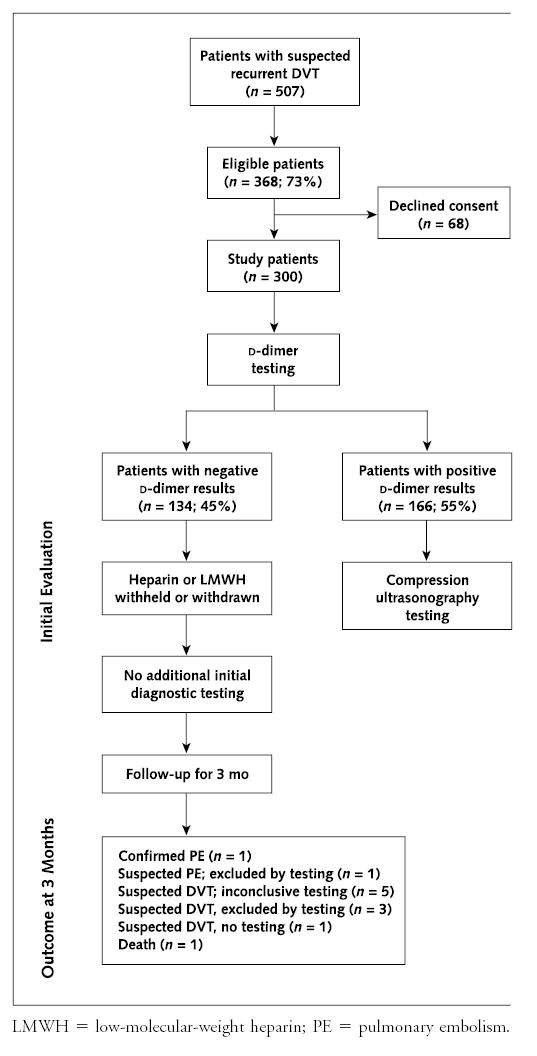
LMWH = low-molecular-weight heparin; PE = pulmonary embolism.
We performed compression ultrasonography in all patients with positive d-dimer test results. This was accomplished by using vein compression and measurement of residual vein diameter beginning at the common femoral vein above the inguinal ligament and moving distally at 1-cm intervals to the trifurcation in the calf. Ultrasonography results were classified as normal if all imaged segments were fully compressible (6, 17). We diagnosed new DVT by the presence of either a new noncompressible venous segment or an increase in vein diameter of greater than 4 mm (compared with the most recent previous ultrasonography result) (17). A registered vascular technologist performed the ultrasonography, and 1 of 2 internists specializing in vascular medicine interpreted the results. These individuals had knowledge of the previous ultrasonography result, if available, and the clinical presentation.
Long-Term Follow-up
We instructed all patients to immediately contact us or go to the emergency department if they had new symptoms or signs of venous thrombosis or pulmonary embolism. Patients were routinely assessed in the clinic or by telephone at 3 months. At this follow-up contact, a history was taken of the interval since study entry. Issues addressed were general health, specific symptoms of venous thromboembolism (including leg pain, tenderness and swelling, chest pain, dyspnea, and hemoptysis), hospitalization, and the use of anticoagulants.
All patients who returned during follow-up with clinically suspected venous thrombosis or pulmonary embolism underwent diagnostic testing. For suspected DVT, compression ultrasonography, impedance plethysmography, or venography was used. For suspected pulmonary embolism, lung scanning, helical computed tomography, or pulmonary angiography was used. The physician seeing the patient selected the diagnostic tests on the basis of clinical presentation and local availability.
The primary outcome measure was a diagnosis of new symptomatic venous thromboembolism, either DVT or pulmonary embolism, confirmed by diagnostic testing during the 3-month follow-up. All patients who fulfilled at least 1 of the diagnostic criteria for DVT or pulmonary embolism were considered to have confirmed venous thromboembolism on follow-up. The 3-month follow-up period was chosen because inadequate management of acute DVT results in a high rate of recurrent venous thromboembolic events during the subsequent 3 months (18–20).
The diagnostic criteria used to confirm or exclude the presence of new DVT or pulmonary embolism on follow-up were defined before the study. The diagnostic criteria used to confirm DVT on follow-up were any one of the following: a new noncompressible segment identified by compression ultrasonography (5, 6, 17), a new constant intraluminal filling defect identified by venography (4), or a newly abnormal impedance plethysmography result (4, 9). Ultrasonography was classified as inconclusive if there was persistent noncompressibility in the same vein segments as the most recent previous ultrasonography result.
The diagnostic criteria used to confirm pulmonary embolism on follow-up were any one of the following: a new high-probability ventilation–perfusion lung scan (a perfusion defect >75% of a segment with ventilation mismatch or in an area of normal findings on chest radiography) (21), a positive helical computed tomography scan (22), a pulmonary angiogram showing a constant intraluminal filling defect (21), or pulmonary embolism found at autopsy.
The diagnostic criteria used to exclude a diagnosis of DVT on follow-up were any of the following: a normal result on compression ultrasonography (that is, all veins fully compressible) (5, 6, 8, 17), a negative venography result (23), or normal results on impedance plethysmography (4, 9). The diagnostic criteria used to exclude a diagnosis of pulmonary embolism on follow-up were any of the following: a normal perfusion lung scan (24) or the absence of new perfusion defects compared with the most recent previous lung scan, a negative pulmonary angiography result (24), or pulmonary embolism excluded by autopsy.
Methodologic Issues and Avoidance of Bias
Care was taken to avoid bias. We avoided selection bias by entering consecutive eligible patients into the study. We avoided bias during the initial testing period by defining criteria for a negative d-dimer result a priori, by prohibiting venography or ultrasonography in patients with negative d-dimer test results, and by withholding heparin and low-molecular-weight heparin therapy from all patients with negative d-dimer test results, regardless of their symptoms. We avoided diagnostic suspicion bias (25) by performing diagnostic testing in all patients who returned during follow-up with new symptoms and signs suggesting DVT or pulmonary embolism. We avoided incorporation bias (25) by not using d-dimer to evaluate patients with symptoms or signs of DVT or pulmonary embolism during long-term follow-up.
The results of all diagnostic tests for DVT or pulmonary embolism performed during follow-up were independently reviewed by 2 persons who did not have knowledge of the patient’s d-dimer result. For each patient who died, the case was independently reviewed by 2 reviewers not involved in the patient’s care. The cause of death was determined without knowledge of the d-dimer or compression ultrasonography results at study entry. Disagreements between reviewers regarding the adjudication of venous thromboembolism on follow-up or the adjudicated cause of death were resolved through independent adjudication by a third reviewer; the majority decision was used as the outcome result.
Statistical Analysis
Before the study, we hypothesized from previous studies (4, 9–13) that the incidence of venous thromboembolism on follow-up in patients with negative d-dimer results would be 2% or less. We specified a priori that, to accept a negative d-dimer as a useful exclusion test, the upper 95% confidence limit for the true incidence of venous thromboembolism on follow-up should be less than 5%.
The exact 95% CIs for the true incidence of venous thromboembolism occurring during follow-up were calculated from the binomial distribution.
Role of the Funding Sources
The study was funded by an Oklahoma Center for Science and Technology Health Research Grant and by a National Institutes of Health, National Heart, Lung, and Blood Institute Research Career Training Award. Diagnostica Stago provided STA-Liatest d-dimer reagents. The funding sources had no role in the design, conduct, and reporting of the study or in the decision to submit the manuscript for publication.
RESULTS
Patients and Study Cohorts
A total of 507 consecutive patients were evaluated between March 2000 and August 2003; 368 (73%) were eligible and 139 were ineligible. Reasons for ineligibility were as follows: signs and symptoms of upper-extremity DVT (n = 5), previous pelvic surgery (n = 4), an indwelling central line (n = 26), pregnancy (n = 10), age younger than 18 (n = 10), enrollment in another study (n = 12), therapeutic low-molecular-weight heparin (n = 25), inability to provide consent (n = 25), incarceration (n = 10), placement of an upper-extremity dialysis graft (n = 6), or ultrasonography inadvertently performed before d-dimer testing (n = 6). Of the 368 eligible patients, 300 (81%) were enrolled (68 declined consent). Table 1 presents the demographic and clinical characteristics of the study sample.
Table 1.
Demographic and Clinical Characteristics*
| Characteristic | Patients (n= 300) | Patients with Negatived-Dimer Results (n= 134) | Patients with Positived-Dimer Results (n= 166) |
|---|---|---|---|
| Demographic characteristics | |||
| Mean age (range), Y | 55 (21–88) | 51 (21–88) | 58 (22–84) |
| Male, n (%) | 167 (56) | 64 (48) | 103 (62) |
| Female, n (%) | 133 (44) | 70 (52) | 63 (38) |
| Inpatients, n (%) | 56 (19) | 13 (10) | 43 (26) |
| Outpatients, n (%) | 244 (81) | 121 (90) | 123 (74) |
| Taking warfarin at presentation, n (%) | 139 (46) | 75 (56) | 64 (39) |
| IVC filter, n (%) | 22 (7) | 10 (8) | 12 (7) |
| Symptoms at presentation, n (%) | |||
| Swelling | 246 (82) | 110 (82) | 136 (82) |
| Pain | 249 (83) | 111 (83) | 138 (83) |
| Tenderness | 248 (83) | 111 (83) | 137 (83) |
| Pain or tenderness or swelling | 300 (100) | 134 (100) | 166 (100) |
| Clinical conditions, n (%) | |||
| Hospitalized in the previous 6 mo | 98 (33) | 38 (28) | 60 (36) |
| Surgery in the previous 6 mo | 57 (19) | 21 (16) | 36 (22) |
| Trauma to the legs in the previous 6 mo | 43 (14) | 16 (12) | 27 (16) |
| Cancer | 56 (19) | 22 (16) | 34 (20) |
| CHF | 28 (9) | 8 (6) | 20 (12) |
| Immobilized in the previous month | 27 (9) | 7 (5) | 20 (12) |
| Pregnancy in the previous year | 8 (3) | 6 (4) | 2 (1) |
| Family history of venous thromboembolism | 69 (23) | 32 (24) | 37 (22) |
CHF = congestive heart failure; IVC = inferior vena cava.
Results of Initial Diagnostic Testing
Of the 300 study patients, 134 (45%) had negative d-dimer results (negative cohort), and 166 had positive d-dimer results (Figure). Of the 166 patients, compression ultrasonography documented new DVT in 54 patients (all had new noncompressible vein segments); ultrasonography results were normal in 79 patients and were inconclusive in 33 patients.
Venous Thromboembolism on Follow-up
No patients were lost to 3-month follow-up. Two patients could not be reached directly (1 in the negative cohort and 1 with new DVT at study entry); in these cases, relatives who had seen the patients recently attested to their good health.
Table 2 summarizes the outcomes on follow-up for 3 months in the negative cohort. Eleven patients in the negative cohort had suspected venous thromboembolism during follow-up ( Table 3). One of 134 patients had confirmed venous thromboembolism (0.75% [CI, 0.02% to 4.09%]). This patient was initially receiving warfarin therapy at the time of presentation but discontinued it and had pulmonary embolism 6 weeks later; a high-probability lung scan confirmed the embolism. Of the remaining 10 patients, diagnostic testing excluded acute venous thromboembolism in 4 patients, was inconclusive in 5 patients, and was not performed in 1 patient. This latter patient was taking warfarin (international normalized ratio, 3.5) at the time of presentation at 61 days, and the physician decided not to perform diagnostic testing. Subsequently, after completion of the 3-month follow-up (101 days after study entry), the patient had new DVT, which was confirmed by compression ultrasonography. This event occurred after the predefined 3-month follow-up period; however, if it is considered in the analysis, the incidence of confirmed venous thromboembolism is 1.5% (CI, 0.18% to 5.3%) (2 of 134 patients). Of the 11 patients in the negative cohort with suspected venous thromboembolism during follow-up, 7 were receiving warfarin treatment at the time of entry into the study.
Table 2.
Summary of Outcomes at 3 Months of Follow-up in the Negative Cohort
| Outcome | Patients (n= 134) [95% CI],n (%) |
|---|---|
| Confirmed venous thromboembolism | 1 (0.75 [0.02–4.09]) |
| Suspected venous thromboembolism; diagnostic testing inconclusive | 5 (3.73) |
| Death | 1 (0.75 [0.02–4.09]) |
Table 3.
Characteristics of Patients in the Negative Cohort with Suspected Recurrent Venous Thromboembolism during Follow-up*
| Patient Study Number | Time to Suspected Event (Days after Study Entry) | Warfarin/INR at Time of Suspected Event | New Risk Factor for Venous Thromboembolism | Diagnostic Test Results† | Additional Anticoagulant Therapy and Outcome‡ |
|---|---|---|---|---|---|
| Suspected DVT | |||||
| R147 | 7 | No warfarin | Hospitalization | US normal | None |
| R086 | 12 | No warfarin | No new risk factor | US inconclusive | None |
| R100 | 28 | No warfarin | No new risk factor | IPG normal | None |
| R108 | 31 | Warfarin/3.6 | Uterine ablation procedure | US inconclusive | None |
| R152 | 38 | No warfarin | No new risk factor | US normal | None |
| R057 | 52 | Warfarin/no INR | No new risk factor | US inconclusive | None |
| R062 | 61 | Warfarin/3.5 | Nonadherence | No testing done | None |
| R025 | 65 | Warfarin/1.5 | No new risk factor | US inconclusive | None |
| R042 | 79 | Warfarin/1.6 | Fall on knees | US inconclusive | LMWH therapy while restoring INR to a value >2.0 |
| Suspected PE | |||||
| R123 | 16 | No warfarin/1.2 | Warfarin therapy withdrawn 72 h before study entry because of bleeding | Lung scan; no new defects | None |
| R228 | 43 | No warfarin | Warfarin therapy discontinued 7 days after study entry | High-probability lung scan | LMWH and warfarin therapy |
DVT = deep venous thrombosis; INR = international normalized ratio; IPG = impedance plethysmography; LMWH = low-molecular-weight heparin; PE = pulmonary embolism; US = ultrasonography.
Inconclusive = abnormal with persistent noncompressibility in the same venous segments as the most recent previous ultrasonography report.
None = no heparin or LMWH therapy was given, and there were no additional symptomatic thromboembolic events at 3 months.
Venous thromboembolism confirmed by diagnostic testing occurred in 4 (2.4%) of the 166 patients with positive d-dimer results at entry (1 with normal compression ultrasonography results at study entry and 3 with DVT at study entry).
Deaths on Follow-up
Six patients died during the study; 1 of these was in the negative cohort. The patient in the negative cohort was a 44-year-old man with a history of congenital venous hypoplasia, recurrent DVT, and hypercholesterolemia. At the time of study entry, this patient was taking warfarin and had an international normalized ratio of 2.0. He also had been prescribed simvastatin but had not taken this medication for several weeks. Eight weeks after study entry, the patient was found deceased at home. Autopsy was not performed, and the death certificate listed the cause of death as acute coronary insufficiency.
If the patient in the negative cohort who died in addition to the 5 patients with inconclusive diagnostic test results ( Table 3) and the 1 patient with recurrent leg symptoms but no diagnostic testing (Figure) are all considered to have venous thromboembolism, the incidence of new venous thromboembolism during follow-up for 3 months in the negative cohort would be 6.0% (CI, 2.6% to 11.4%) (8 of 134 patients).
The causes of death in the 5 patients with positive d-dimer results were acute myocardial infarction (6 days after study entry), documented gram-negative sepsis (17 days after study entry), metastatic parotid cancer (11 weeks after study entry), acute myelocytic leukemia (12 weeks after study entry), and septicemia (9 weeks after study entry).
DISCUSSION
In patients with suspected recurrent DVT, an automated assay for d-dimer, the STA-Liatest d-di, when used alone, may provide a simple method for excluding acute recurrent DVT. There was a low incidence (0.75% [CI, 0.02% to 4.09%]) of confirmed symptomatic venous thromboembolism after 3 months of follow-up in patients with a negative d-dimer result at presentation. The STA-Liatest d-di assay result was negative at presentation in 45% of patients (CI, 39.0% to 50.5%), providing a high clinical utility in avoiding the need for additional diagnostic testing and heparin treatment. The cost to the third-party payer for the STA-Liatest d-di assay is approximately $20; ultrasonography usually costs more than $200.
The acceptable upper limit for the incidence of venous thromboembolism on follow-up in patients with a negative d-dimer test result remains a clinical judgment in the individual patient. The prognosis on follow-up in our patients with negative d-dimer test results is similar to the prognosis of patients with negative results on combined impedance plethysmography and fibrinogen leg scanning (4); it is also similar to the prognosis of patients with negative venography results (23). We believe that the assay for d-dimer is a useful addition to the physician’s options for patients with suspected recurrent DVT when balanced against the limitations and risks of the currently available alternative diagnostic approaches and the risk of anticoagulant therapy (26).
Wells and colleagues (27) used d-dimer in combination with a clinical model of pretest probability to withhold ultrasonography testing and anticoagulant treatment in patients classified as unlikely to have DVT by the clinical model. Most patients (82%) in that study (27) had suspected first-episode venous thromboembolism. A history of DVT is a criterion in the clinical model that weighs strongly against pretest classification as unlikely (27). Therefore, according to the clinical model of Wells, many patients with suspected recurrent DVT will not be classified as unlikely, and ultrasonography is still required for these patients. In contrast, our study focused entirely on patients with suspected recurrent DVT and used the d-dimer test result to determine management without the need for a clinical model of pretest probability.
Our study has several limitations. First, it was not possible to definitively exclude or confirm the presence of acute recurrent venous thromboembolism on follow-up in 7 patients in the negative cohort. This represents a practical limitation of the available diagnostic methods and the lack of a diagnostic reference standard for recurrent DVT. Our study used state-of-the-art diagnostic methods. Even if these 7 patients in the negative cohort are considered to be positive for venous thromboembolism, the incidence remains low at 6.0% (8 of 134 patients). This incidence is much lower than the expected 20% incidence of symptomatic recurrent thromboembolic events during the 3 months after diagnosis of acute DVT for patients who received ineffective treatment (18–20). Therefore, if many patients in the negative cohort actually had DVT at study entry, we would have expected a considerably higher incidence of venous thromboembolism during the 3-month follow-up period. Second, 56% of patients in the negative cohort were receiving warfarin therapy at the time of presentation. Again, this represents a practical reality for these patients with a history of DVT. We chose to include patients receiving warfarin in order to make the findings generalizable to clinical practice. It is possible that warfarin treatment may have resulted in false-negative d-dimer test results. Additional studies should be done to definitively determine whether the validity of a negative d-dimer result for excluding acute recurrent DVT is influenced by warfarin anticoagulation. Third, our results allow us to estimate the incidence of symptomatic venous thromboembolism during 3 months after a negative d-dimer result to range from approximately 1% to 11%. Additional studies in more patients are required to provide a more precise estimate of the true incidence. Finally, our study was done at an academic health center, and the generalizability of our results to other clinical settings requires additional evaluation.
In conclusion, the automated assay for d-dimer, STA-Liatest d-di, seems to provide a simple method with high clinical utility for excluding acute recurrent DVT in patients in whom this diagnosis is suspected.
Acknowledgments
The authors thank Kevin Dahlin, Kyle Enfield, Janette Epperly, Jay Heath, Brenda Holliefield, Cindy Jones, Peggy Powell, and Penny Razo-Mosier for their assistance with this project.
Footnotes
Grant Support: By an Oklahoma Center for the Advancement of Science and Technology (OCAST) Health Research Grant (HR0-052) and in part by a National Institutes of Health, National Heart, Lung, and Blood Institute Research Career Training Award (K23) (HL04200-03). STA-Liatest d-dimer reagents were provided by Diagnostica Stago.
Potential Financial Conflicts of Interest: Grants received: S.W. Rathbun (Diagnostica Stago); Other: S.W. Rathbun (reagents for d-dimer were provided by Diagnostica Stago).
Current author addresses and author contributions are available at www.annals.org
Current Author Addresses :Drs. Rathbun and Whitsett: Department of Medicine, WP 3120, University of Oklahoma Health Sciences Center, 920 Stanton L. Young Boulevard, Oklahoma City, OK 73104.
Dr. Raskob: University of Oklahoma Health Sciences Center, College of Public Health, 801 Northeast 13th Street, Room 139, Oklahoma City, OK 73104.
Author Contributions: Conception and design: S.W. Rathbun, T.L. Whitsett, G.E. Raskob.
Analysis and interpretation of the data: S.W. Rathbun, T.L. Whitsett, G.E. Raskob.
References
- 1.Silverstein MD, Heit JA, Mohr DN, Petterson TM, O’Fallon WM, Melton LJ., 3rd Trends in the incidence of deep vein thrombosis and pulmonary embolism: a 25-year population-based study. Arch Intern Med. 1998;158:585–93. doi: 10.1001/archinte.158.6.585. [PMID: 9521222] [DOI] [PubMed] [Google Scholar]
- 2.Anderson FA, Jr, Wheeler HB, Goldberg RJ, Hosmer DW, Patwardhan NA, Jovanovic B, et al. A population-based perspective of the hospital incidence and case-fatality rates of deep vein thrombosis and pulmonary embolism. The Worcester DVT Study. Arch Intern Med. 1991;151:933–8. [PMID: 2025141] [PubMed] [Google Scholar]
- 3.Koopman MM, Buller HR, ten Cate JW. Diagnosis of recurrent deep vein thrombosis. Haemostasis. 1995;25:49–57. doi: 10.1159/000217143. [PMID: 7896222] [DOI] [PubMed] [Google Scholar]
- 4.Hull RD, Carter CJ, Jay RM, Ockelford PA, Hirsch J, Turpie AG, et al. The diagnosis of acute, recurrent, deep-vein thrombosis: a diagnostic challenge. Circulation. 1983;67:901–6. doi: 10.1161/01.cir.67.4.901. [PMID: 6825247] [DOI] [PubMed] [Google Scholar]
- 5.Huisman MV, Beaumont-Koopman MM. The diagnosis of recurrent deep-vein thrombosis. In: Hull Rd, Raskob GE, and Pineo GF, eds. Venous Thromboembolism: An Evidence-Based Atlas. Armonk, NY: Futura Publishing; 1996:148-XXX.
- 6.Heijboer H, Jongbloets LM, Buller HR, Lensing AW, ten Cate JW. Clinical utility of real-time compression ultrasonography for diagnostic management of patients with recurrent venous thrombosis. Acta Radiol. 1992;33:297–300. [PMID: 1633039] [PubMed] [Google Scholar]
- 7.Murphy TP, Cronan JJ. Evolution of deep venous thrombosis: a prospective evaluation with US. Radiology. 1990;177:543–8. doi: 10.1148/radiology.177.2.2217798. [PMID: 2217798] [DOI] [PubMed] [Google Scholar]
- 8.Prandoni P, Lensing AW, Prins MH, Bernardi E, Marchiori A, Bagatella P, et al. Residual venous thrombosis as a predictive factor of recurrent venous thromboembolism. Ann Intern Med. 2002;137:955–60. doi: 10.7326/0003-4819-137-12-200212170-00008. [PMID: 12484710] [DOI] [PubMed] [Google Scholar]
- 9.Huisman MV, Buller HR, ten Cate JW. Utility of impedance plethysmography in the diagnosis of recurrent deep-vein thrombosis. Arch Intern Med. 1988;148:681–3. [PMID: 3341869] [PubMed] [Google Scholar]
- 10.Bates SM, Lister-James J, Julian JA, Taillefer R, Moyer BR, Ginsberg JS. Imaging characteristics of a novel technetium Tc 99m-labeled platelet glycoprotein IIb/IIIa receptor antagonist in patients with acute deep vein thrombosis or a history of deep vein thrombosis. Arch Intern Med. 2003;163:452–6. doi: 10.1001/archinte.163.4.452. [PMID: 12588204] [DOI] [PubMed] [Google Scholar]
- 11.Stein PD, Hull RD, Patel KC, Olson RE, Ghali WA, Brant R, et al. D-dimer for the exclusion of acute venous thrombosis and pulmonary embolism: a systematic review. Ann Intern Med. 2004;140:589–602. doi: 10.7326/0003-4819-140-8-200404200-00005. [PMID: 15096330] [DOI] [PubMed] [Google Scholar]
- 12.Bernardi E, Prandoni P, Lensing AW, Agnelli G, Guazzaloca G, Scannapieco G, et al. D-dimer testing as an adjunct to ultrasonography in patients with clinically suspected deep vein thrombosis: prospective cohort study. The Multi-centre Italian D-dimer Ultrasound Study Investigators Group. BMJ. 1998;317:1037–40. doi: 10.1136/bmj.317.7165.1037. [PMID: 9774286] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Perrier A, Desmarais S, Miron MJ, de Moerloose P, Lepage R, Slosman D, et al. Non-invasive diagnosis of venous thromboembolism in outpatients. Lancet. 1999;353:190–5. doi: 10.1016/S0140-6736(98)05248-9. [PMID: 9923874] [DOI] [PubMed] [Google Scholar]
- 14.Bates SM, Kearon C, Crowther M, Linkins L, O’Donnell M, Douketis J, et al. A diagnostic strategy involving a quantitative latex D-dimer assay reliably excludes deep venous thrombosis. Ann Intern Med. 2003;138:787–94. doi: 10.7326/0003-4819-138-10-200305200-00006. [PMID: 12755550] [DOI] [PubMed] [Google Scholar]
- 15.van der Graaf F, van den Borne H, van der Kolk M, de Wild PJ, Janssen GW, van Uum SH. Exclusion of deep venous thrombosis with D-dimer testing—comparison of 13 D-dimer methods in 99 outpatients suspected of deep venous thrombosis using venography as reference standard. Thromb Haemost. 2000;83:191–8. [PMID: 10739371] [PubMed] [Google Scholar]
- 16.Oger E, Leroyer C, Bressollette L, Nonent M, Le Moigne E, Bizais Y, et al. Evaluation of a new, rapid, and quantitative D-Dimer test in patients with suspected pulmonary embolism. Am J Respir Crit Care Med. 1998;158:65–70. doi: 10.1164/ajrccm.158.1.9710058. [PMID: 9655708] [DOI] [PubMed] [Google Scholar]
- 17.Prandoni P, Cogo A, Bernardi E, Villalta S, Polistena P, Simioni P, et al. A simple ultrasound approach for detection of recurrent proximal-vein thrombosis. Circulation. 1993;88:1730–5. doi: 10.1161/01.cir.88.4.1730. [PMID: 8403319] [DOI] [PubMed] [Google Scholar]
- 18.Hull R, Delmore T, Genton E, Hirsh J, Gent M, Sackett D, et al. Warfarin sodium versus low-dose heparin in the long-term treatment of venous thrombosis. N Engl J Med. 1979;301:855–8. doi: 10.1056/NEJM197910183011602. [PMID: 384248] [DOI] [PubMed] [Google Scholar]
- 19.Hull RD, Raskob GE, Hirsh J, Jay RM, Leclerc JR, Geerts WH, et al. Continuous intravenous heparin compared with intermittent subcutaneous heparin in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1986;315:1109–14. doi: 10.1056/NEJM198610303151801. [PMID: 3531862] [DOI] [PubMed] [Google Scholar]
- 20.Brandjes DP, Heijboer H, Buller HR, de Rijk M, Jagt H, ten Cate JW. Acenocoumarol and heparin compared with acenocoumarol alone in the initial treatment of proximal-vein thrombosis. N Engl J Med. 1992;327:1485–9. doi: 10.1056/NEJM199211193272103. [PMID: 1406880] [DOI] [PubMed] [Google Scholar]
- 21.Value of the ventilation/perfusion scan in acute pulmonary embolism. Results of the prospective investigation of pulmonary embolism diagnosis (PIOPED). The PIOPED Investigators. JAMA. 1990;263:2753–9. doi: 10.1001/jama.1990.03440200057023. [PMID: 2332918] [DOI] [PubMed] [Google Scholar]
- 22.Rathbun SW, Raskob GE, Whitsett TL. Sensitivity and specificity of helical computed tomography in the diagnosis of pulmonary embolism: a systematic review. Ann Intern Med. 2000;132:227–32. doi: 10.7326/0003-4819-132-3-200002010-00009. [PMID: 10651604] [DOI] [PubMed] [Google Scholar]
- 23.Hull R, Hirsh J, Sackett DL, Taylor DW, Carter C, Turpie AG, et al. Clinical validity of a negative venogram in patients with clinically suspected venous thrombosis. Circulation. 1981;64:622–5. doi: 10.1161/01.cir.64.3.622. [PMID: 7261292] [DOI] [PubMed] [Google Scholar]
- 24.Kruip MJ, Leclercq MG, van der Heul C, Prins MH, Buller HR. Diagnostic strategies for excluding pulmonary embolism in clinical outcome studies. A systematic review. Ann Intern Med. 2003;138:941–51. doi: 10.7326/0003-4819-138-12-200306170-00005. [PMID: 12809450] [DOI] [PubMed] [Google Scholar]
- 25.Sackett DL. Bias in analytic research. J Chronic Dis. 1979;32:51–63. doi: 10.1016/0021-9681(79)90012-2. [PMID: 447779] [DOI] [PubMed] [Google Scholar]
- 26.Linkins LA, Choi PT, Douketis JD. Clinical impact of bleeding in patients taking oral anticoagulant therapy for venous thromboembolism: a meta-analysis. Ann Intern Med. 2003;139:893–900. doi: 10.7326/0003-4819-139-11-200312020-00007. [PMID: 14644891] [DOI] [PubMed] [Google Scholar]
- 27.Wells PS, Anderson DR, Rodger M, Forgie M, Kearon C, Dreyer J, et al. Evaluation of D-dimer in the diagnosis of suspected deep-vein thrombosis. N Engl J Med. 2003;349:1227–35. doi: 10.1056/NEJMoa023153. [PMID: 14507948] [DOI] [PubMed] [Google Scholar]


