Abstract
Functional interactions between classical cadherins and the actin cytoskeleton involve diverse actin activities, including filament nucleation, cross-linking, and bundling. In this report, we explored the capacity of Ena/VASP proteins to regulate the actin cytoskeleton at cadherin-adhesive contacts. We extended the observation that Ena/vasodilator-stimulated phosphoprotein (VASP) proteins localize at cell–cell contacts to demonstrate that E-cadherin homophilic ligation is sufficient to recruit Mena to adhesion sites. Ena/VASP activity was necessary both for F-actin accumulation and assembly at cell–cell contacts. Moreover, we identified two distinct pools of Mena within individual homophilic adhesions that cells made when they adhered to cadherin-coated substrata. These Mena pools localized with Arp2/3-driven cellular protrusions as well as at the tips of cadherin-based actin bundles. Importantly, Ena/VASP activity was necessary for both modes of actin activity to be expressed. Moreover, selective depletion of Ena/VASP proteins from the tips of cadherin-based bundles perturbed the bundles without affecting the protrusive F-actin pool. We propose that Ena/VASP proteins may serve as higher order regulators of the cytoskeleton at cadherin contacts through their ability to modulate distinct modes of actin organization at those contacts.
INTRODUCTION
Cadherins are a family of calcium-dependent cell–cell adhesion molecules that regulate the actin cytoskeleton. On productive ligation of their adhesive ectodomains, classical cadherins activate cellular responses necessary for a range of morphogenetic processes, including cell sorting and tissue cohesion (Tepass et al., 2000), cell-upon-cell locomotion (Brieher and Gumbiner, 1994), and the coordination of cell migration in dorsal closure and wound repair (Danjo and Gipson, 1998). These morphogenetic responses likely reflect complex functional and biochemical interactions between the cadherin, its associated catenins, cell signaling pathways, and the cytoskeleton. Dynamic activity of the actin cytoskeleton, in particular, must be coordinated with surface adhesion to provide local protrusive force and mechanical stability at cell–cell contacts (Takeichi, 1991; Gumbiner, 1996; Yap et al., 1997). The challenge, then, is to define key molecular regulators responsible for these functional interrelationships.
The capacity for cadherins to regulate actin was first suggested by the extensive alterations in actin filament distribution that occur when epithelial cells adhere to one another (Yonemura et al., 1995; Adams et al., 1996). It subsequently became evident that multiple forms of actin activity occur at cadherin contacts, including de novo nucleation and cross-linking, that must presumably be stringently coordinated with the dynamic state of adhesive contacts (Yonemura et al., 1995; Adams et al., 1996; Vasioukhin et al., 2000; Waterman-Storer et al., 2000; Kobielak et al., 2004; Verma et al., 2004). Indeed, a growing corpus of actin–regulatory proteins have been identified at cadherin-based cell–cell contacts. These include actin nucleators, such as the Arp2/3 complex and formin-1 (Kovacs et al., 2002b; Kobielak et al., 2004); proteins that can modulate actin assembly, such as cortactin (Helwani et al., 2004); and potential actin-bundling proteins such as α-catenin and vinculin (Nagafuchi et al., 1991; Rimm et al., 1995; Watabe-Uchida et al., 1998; Vasioukhin et al., 2000) as well as a range of other proteins, such as KLEIP (Hara et al., 2004), that may influence actin organization.
It is increasingly apparent that actin organization and dynamics are controlled by hierarchies of regulatory molecules. In addition to the core machinery responsible for actin assembly, turnover, and cross-linking, a range of higher order regulators have been reported that may coordinate different modes of actin organization (Pollard and Borisy, 2003). One such set of potential regulators are the Ena/vasodilator-stimulated phosphoprotein (VASP) proteins (Gertler et al., 1990, 1995; Krause et al., 2003). In mammals, these regulators include Mena, the homologue of Drosophila Ena, VASP, and EVL (Reinhard et al., 1992; Gertler et al., 1996). All members of the Ena/VASP family have a tripartite structure, consisting of an EVH1 domain, a central proline-rich domain, and a terminal EVH2 domain (reviewed in Krause et al., 2003). The EVH1 domain binds to FP4 motifs found in proteins such as vinculin, ActA, and zyxin. The central proline-rich region binds to SH3 domains and the actin binding protein profilin, whereas the terminal EVH2 domain supports tetramerization and F-actin binding. Both the EVH1 and EVH2 domains mediate Ena/VASP targeting within cells. Ena/VASP proteins localize to the tips of actin bundles and to the leading edges of migratory fibroblasts (Gertler et al., 1996; Rottner et al., 1999; Bear et al., 2000) and in growth cones (Lanier et al., 1999; Goh et al., 2002; Lebrand et al., 2004), where they regulate filament elongation and branching and consequently force production and cell surface protrusiveness (Bear et al., 2002).
Several recent observations suggest that Ena/VASP proteins also affect cadherin-based actin organization. Ena/VASP proteins are found at cell–cell contacts between follicular cells of the Drosophila egg chamber (Baum and Perrimon, 2001) and between cultured murine keratinocytes (Vasioukhin et al., 2000), their localization in the latter case depending on the integrity of those contacts. In Drosophila (Baum and Perrimon, 2001), ena mutants decreased F-actin throughout cell cortices, including that found at cell–cell contacts. Furthermore, a genetic interaction between abelson and ena controls the balance of actin assembly within Drosophila epithelial cells. Abelson mutants promote accumulation of F-actin at the apical surfaces of cells at the expense of F-actin at cell–cell contacts in an ena-dependent manner (Grevengoed et al., 2001; Grevengoed et al., 2003). Nonetheless, how these genetic effects might be expressed at a cellular level to affect actin cytoskeletal regulation by cadherins remains to be elucidated. In this study, we therefore sought to further explore the potential for Ena/VASP proteins to mediate higher order regulation of the actin cytoskeleton by E-cadherin.
MATERIALS AND METHODS
Cell Culture, Transfections, and hE/Fc
hE-chinese hamster ovary (CHO) cells and MCF-7 cells were described previously (Kovacs et al., 2002b; Paterson et al., 2003). MVD7 and D7-FLM cells were cultured as described previously (Bear et al., 2000). hE/Fc, a recombinant protein bearing the complete ectodomain of E-cadherin fused to the Fc region of human IgG, was purified and coated onto either latex beads (6 μm; Sigma-Aldrich, St, Louis, MO) or onto glass coverslips as described previously (Kovacs et al., 2002b).
Plasmids
Enhanced green fluorescent protein (EGFP)-Mena was described previously (Bear et al., 2000). For transient expression in mammalian cells, the coding sequences for EGFP-Mena, EGFP AP4-cyto, EGFP FP4-cyto, EGFP AP4-mito, and EGFP FP4-mito were subcloned from pMSCV (Bear et al., 2000) into pCDNA3.1(–) by digestion with EcoR1 and HindIII. For the creation of stable cell lines, the inserts was moved to an intermediary plasmid, pTRE2, by digesting with Not1 and HindIII; subsequent digestion with EcoRV generated the insert sequence for ligation into pIRESpuro (Clontech, Mountain View, CA). The Arp3-EGFP vector, constructed by Dr. .Matt Welch, was a gift from Dr. Dorothy Schafer (Washington University, St. Louis, MO) (Schafer et al., 1998). The EGFP-IRSp53 construct was a kind gift from Drs. H. Miki and T. Takenawa (University of Tokyo, Tokyo, Japan) (Miki et al., 2000). The plasmid encoding full-length human E-cadherin was a gift from Dr. Cara Gottardi and has been described previously (Kovacs et al., 2002b). All transfections were performed using Lipofectamine (Invitrogen, Carlsbad, CA) in accordance with the manufacturer's instructions.
Antibodies and Immunochemicals
Primary antibodies and immunochemicals were as follows: 1) Rabbit polyclonal antibody (pAb) against Mena (Bear et al., 2000). 2) Mouse monoclonal antibody (mAb) raised against the C-terminal domain of Mena (BD Transduction Laboratories, Lexington, KY). 3) Mouse mAb raised against the cytoplasmic tail of human E-cadherin (BD Transduction Laboratories). 4) Rabbit pAb raised against the cytoplasmic tail of mouse E-cadherin. In Western blots this antibody recognized a peptide at ∼120 kDa in both hE-CHO and MCF-7 cell lysates but not in lysates from cadherin-deficient parental CHO cells (our unpublished data). Prebleed samples failed to detect any bands in Western blots. 5) Mouse mAb HECD1 raised against the ectodomain of human E-cadherin (provided by Dr. M. Wheelock, University of Nebraska, Omaha, NE, with the permission of Dr. M. Takeichi). 6) Rabbit pAb directed against the ectodomain of human E-cadherin (Helwani et al., 2004). 7) Mouse mAb 4F11 directed against the repeat region of cortactin (a kind gift from Dr. Scott Weed, Mary Babb Randolph Cancer Research Center, West Virginia University, Morgantown, WV). 8) F-actin was visualized using phalloidin conjugated to tetramethylrhodamine B isothiocyanate (Sigma-Aldrich), Alexa 488 (Molecular Probes, Eugene, OR) or Alexa 350 (Molecular Probes). 9) Mouse mAb directed against vinculin (Sigma-Aldrich). 10) Anti-green fluorescent protein (GFP) mAb (Roche Diagnostics, Indianapolis, IN) and rabbit anti-GFP pAb (Molecular Probes). 11) Alexa 594-labeled G-actin (Molecular Probes). Secondary antibodies were species-specific and conjugated with Alexa 350, Alexa 488, or Texas Red (Molecular Probes).
Immunofluorescence Microscopy
Specimens were fixed for immunofluorescence microscopy as described previously (Kovacs et al., 2002a,b). Epi-illumination fluorescence microscopy was performed using Olympus IX-81 microscopes equipped with 100 and 60× 1.4 numerical aperture objectives. Images were acquired using Hamamatsu Orca 1-ER cameras driven by MetaMorph software version 5.0. For live cell microscopy cells were plated onto hE/Fc-coated substrata in an incubation chamber maintained at 37°C. Image collection was commenced when cell spreading had begun. Image processing was performed in Adobe Photoshop version 7 (Adobe Systems, Mountain View, CA).
Drug Inhibition Studies
Drug inhibition studies were performed by allowing MCF-7 cells to spread on hE/Fc for 60 min. Cytochalasin D (25 nM final concentration from 1 mM stock in dimethyl sulfoxide [DMSO]) or Y27632 [10 μM] were then added, and the cells were incubated for another 30 min before fixation. Control cells were incubated in 1:4000 DMSO.
F-Actin Accumulation Assay
F-actin accumulation at cell contacts was measured using the line scan function in MetaMorph version 5.0 (Universal Imaging, Downingtown, PA) to quantitate the intensity of phalloidin fluorescence at cell–cell contacts. In total, 40 lines, each 220 pixels in length, were drawn through each contact, and the average pixel intensity for each position along the line was determined. A minimum of 20 junctions were analyzed per experiment, and the areas under the curves were determined using Excel (Microsoft, Redmond, WA). Experiments were repeated three times.
G-Actin Incorporation Assay
G-actin incorporation assays were performed in saponin-permeabilized live cells as described previously (Kovacs et al., 2002b). Fluorescence intensity of G-actin incorporation was measured by tracing the contacts of cells and measuring pixel intensity with MetaMorph. As for actin accumulation assays, contacts between cells both expressing GFP were chosen for analysis.
RESULTS
Homophilic Ligation Recruits Mena to Cadherin-adhesive Contacts
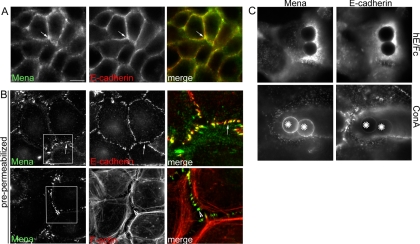
We found that Mena localized at E-cadherin-enriched contacts between MCF-7 mammary epithelial cells (Figure 1A), as has also been observed in Drosophila embryos and primary keratinocytes (Vasioukhin et al., 2000; Grevengoed et al., 2001). However, whereas E-cadherin distributed in an extensive linear pattern throughout the contacts, Mena staining was less continuous, often being found in prominent puncta (Figure 1A) that were particularly evident in cells extracted with nonionic detergents before fixation (Figure 1B). Mena puncta often coincided both with detergent-resistant puncta of E-cadherin and with the termini of perijunctional actin bundles (Figure 1B). Mena staining at contacts was also more prominent as cells reassembled contacts after chelation of extracellular calcium (Supplemental Figure 1) as well as in subconfluent islands of cells (our unpublished data). This suggested that Mena might preferentially accumulate in specific regions within cadherin contacts in response to cellular context.
Figure 1.
Mena localizes to cadherin-adhesive contacts. (A and B) Mena localizes at epithelial cell–cell contacts. MCF-7 cell monolayers were costained for Mena, E-cadherin, or F-actin (phalloidin). (A) In cells permeabilized after fixation, Mena accumulated with E-cadherin at cell–cell contacts (arrow), but it did not precisely colocalize with cadherin. (B) In cells extracted with Triton X-100 before fixation, Mena costained with E-cadherin in prominent puncta at the cell–cell contacts (arrows). These Mena-enriched cadherin puncta formed termini for prominent perijunctional actin bundles (arrowheads). Overlay panels on the right of each row represent magnified views of regions identified by the boxes. (C) Cadherin ligation is sufficient to recruit Mena to the cell surface. Latex beads coated with either hE/Fc or ConA were allowed to adhere to the dorsal surface of hE-CHO cells for 90 min. Cells were then costained for Mena and E-cadherin. Both proteins show an intense accumulation around sites of adhesion with hE/Fc-coated beads but not with control ConA beads. Bar (A), 20 μm.
E-cadherin adhesion can regulate cytoskeletal organization by recruiting actin regulatory proteins to the cell cortex (Kovacs et al., 2002b; Verma et al., 2004). To test whether the localization of Mena at cell–cell contacts might reflect a similar instructive influence of E-cadherin, we first compared the distribution of Mena in monolayers of CHO cells stably expressing E-cadherin (hE-CHO cells) and parental CHO cells, which do not express classical cadherins (Supplemental Figure 2). Similar to MCF-7 cells, Mena and E-cadherin coaccumulated in puncta at cell–cell contacts between hE-CHO cells. In contrast, less Mena staining was seen at contacts between parental CHO cells, where instead it principally stained at the free margins of cells and at the termini of actin stress fibers, presumably at focal adhesions. The expression of E-cadherin thus seemed to promote Mena recruitment to cell–cell contacts.
We then tested whether cadherin homophilic ligation alone could recruit Mena to sites of adhesion. For these experiments, we used latex beads coated with a recombinant cadherin ligand (hE/Fc) that make spatially defined cadherin adhesions when they bind to the dorsal surfaces of hE-CHO cells. As reported previously (Kovacs et al., 2002b; Gavard et al., 2004; Helwani et al., 2004; Verma et al., 2004), hE/Fc and similar recombinant ligands support cadherin-specific adhesion and activate cell signaling. Both cellular E-cadherin and Mena efficiently coaccumulated at sites of adhesion with hE/Fc-coated beads (Figure 1C). In contrast, control beads coated with Concanavalin A (ConA) bound robustly to the cell surface but failed to recruit either Mena or E-cadherin (Figure 1C). Together, these observations identify Mena as one of several functionally distinct actin regulators that can be recruited to adhesions in response to E-cadherin homophilic ligation alone, a process likely to contribute significantly to the junctional localization of Mena that we observed at cell–cell contacts.
Ena/VASP Protein Activity Regulates Actin Filament Density and Assembly at Epithelial Cell–Cell Contacts
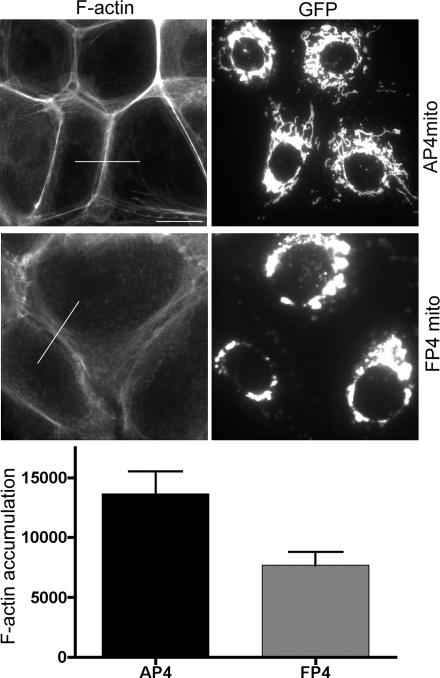
We next tested the impact of Ena/VASP activity on the actin cytoskeleton at native MCF-7 cell–cell contacts. For these studies, we transiently expressed a mitochondrially targeted construct that contains four FP4 motifs from Listeria ActA (EGFP-FP4-mito). By binding their EVH1 domains, this construct sequesters Mena and other Ena/VASP proteins to mitochondrial surfaces and away from their normal cellular locations (Bear et al., 2000), including cell–cell contacts (our unpublished data). As a control, we used a construct with tandem AP4 motifs that is targeted to mitochondria but does not bind Ena/VASP proteins (EGFP-AP4-mito). Fluorescent phalloidin staining seemed significantly reduced at contacts between cells expressing EGFP-FP4-mito compared with either cells expressing EGFP-AP4-mito or untransfected control cells (Figure 2). This evident reduction in F-actin content was confirmed by quantitation of fluorescence intensity at cell–cell contacts (Figure 2).
Figure 2.
Ena/VASP proteins modulate the actin cytoskeleton at cell contacts MCF-7 cells were transiently transfected with EGFP-AP4-mito or EGFP-FP4-mito constructs and stained for GFP and F-actin (phalloidin). Cells expressing the transgenes were identified by EGFP expression and cell–cell contacts defined with E-cadherin staining (our unpublished data). F-actin staining was less prominent between cells that expressed EGFP-FP4-mito compared with control cells. F-actin accumulation between cells expressing transgenes was measured using the line scan function in MetaMorph to quantitate phalloidin fluorescence intensity; representative lines for analysis are shown. Data are means ± SEM (arbitrary fluorescence units; n = 20; p = 0.006; Student's t test); a representative of three independent experiments is shown. Bar, 20 μm.
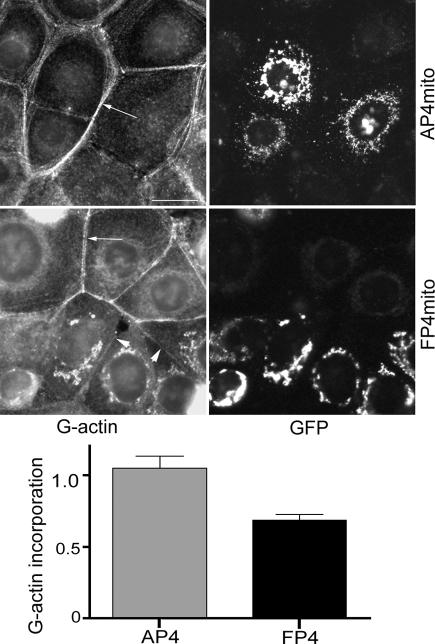
Ena/VASP proteins may regulate the actin cytoskeleton by a number of mechanisms, including both modulation of actin filament assembly (Skoble et al., 2001) as well by contributing to the formation of actin filament bundles (Svitkina et al., 2003). Cadherin-based cell–cell contacts are sites of actin assembly that can be detected by incorporation of fluorescently labeled G-actin (Kovacs et al., 2002b; Verma et al., 2004). To determine whether the observed changes in F-actin content at cell–cell contacts might reflect changes in actin assembly, we performed G-actin incorporation assays in these cells (Figure 3). Live cells were lightly permeabilized before incubation with Alexa 594-conjugated G-actin and then washed and fixed to identify the incorporated pool of G-actin. Control untransfected cells or cells expressing EGFP-AP4-mito showed clear fluorescent labeling at the cell–cell contacts themselves as well as in perijunctional bundles (Figure 3). In contrast, G-actin incorporation at the cellular contacts as well as in perijunctional bundles was much less intense between cells expressing EGFP-FP4-mito, being reduced by ∼40% compared with the negative controls as assessed by quantitation of fluorescence intensity (Figure 3). Together, these findings confirmed that Ena/VASP proteins act to regulate the actin cytoskeleton at cadherin-based cell–cell contacts. They further implied that this regulation occurs, at least in part, through control of actin filament assembly.
Figure 3.
Ena/VASP proteins regulate actin assembly at cadherin-based cell–cell contacts. Actin assembly was measured by incorporation of fluorescent G-actin in live cells. MCF-7 cells were transiently transfected with EGFP-AP4-mito or EGFP-FP4-mito. Cells were lightly permeabilized and exposed to Alexa 594-conjugated G-actin before fixation. Transfected cells were identified by EGFP expression. Fluorescence intensity of incorporated G-actin at cell contacts was measured using the ROI tool in MetaMorph. Incorporation of Alexa 594-G-actin into perijunctional bundles (arrows) and at cell–cell contacts (arrowheads) was significantly reduced in cells expressing EGFP-FP4-mito compared with controls. Alexa 594-G-actin that accumulated at mitochondria in cells expressing EGFP-FP4-mito but without corresponding accumulation of phalloidin staining (Figure 2) may represent binding of actin monomers or short filaments that are not recognized by phalloidin. Data are means ± SEM (n = 20; p = 0.0002; Student's t test); a representative of three independent experiments is shown. Bar, 20 μm.
Mena Localizes in Two Distinct Patterns within Individual Cadherin Homophilic Adhesions
Given the suggestion that Mena might preferentially accumulate at specific regions within cell–cell contacts (Figure 1), we sought to examine more closely the spatial relationship between Mena and E-cadherin at adhesive contacts. Because homophilic ligation sufficed to recruit Mena, we used cadherin planar adhesion assays for these experiments (Figure 4). Cells expressing E-cadherin adhere to hE/Fc-coated substrata in a cadherin-dependent manner with no detectable contribution from integrins (Kovacs et al., 2002b). As they adhere, cells extend contact zones through the generation of broad cadherin-based lamellipodia, thereby providing an optically amenable way to assess the subcellular localization of molecules within individual homophilic adhesions.
Figure 4.
Mena accumulates in two distinct pools at the cadherin homophilic-adhesive interface. (A) hE-CHO cells were plated onto hE/Fc and allowed to adhere for 60 min, and then they were fixed and costained for Mena and E-cadherin. E-cadherin localized in clusters of varying size throughout the adhesive interface. Mena accumulated both in a linear pattern at the very outer margins of cadherin-based lamellipodia (arrowheads) and with large streak-like clusters (macroclusters) of E-cadherin located proximal to the outer margins (arrows). Higher magnification views of the regions enclosed by boxes are shown in the bottom right-hand panel. (B) hE-CHO cells were transiently transfected with EGFP-Mena and imaged using time-lapse videomicroscopy. EGFP-Mena also displayed localization at the protruding outer margins of cells (arrowheads) as well as in more proximal macroclusters (arrows). EGFP-Mena at the outer margins displayed dynamic movement, typically localizing to protruding margins, whereas Mena in macroclusters showed less dynamic lateral mobility. The original position of the outer margin (0 s) is outlined in the 80-s frame to illustrate the degree of protrusion. The full movie is displayed as Supplemental Movie 1. Bar (A), 10 μm.
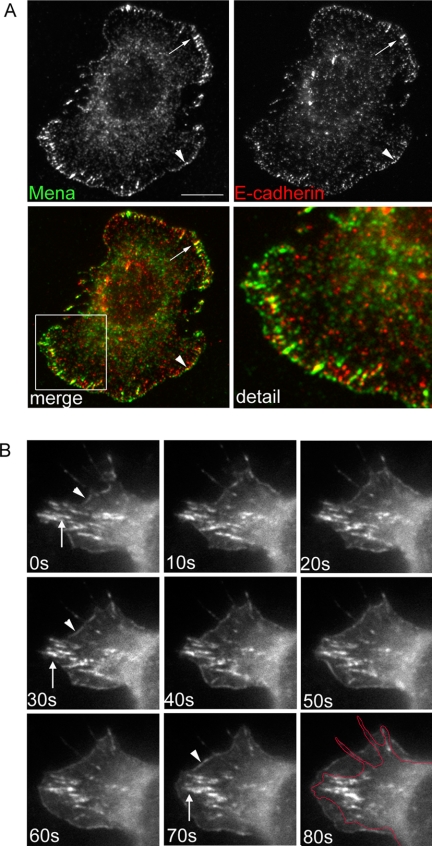
As described previously (Kovacs et al., 2002b; Gavard et al., 2004; Helwani et al., 2004), cellular E-cadherin was found distributed throughout the adhesive contact zones in these assays, typically forming lateral clusters of varying size, both fine puncta and large streaks and clusters (which we refer to as “macroclusters”; Figure 4A)1. Cadherin macroclusters superficially resemble integrin focal adhesions, but they are integrin independent (Kovacs et al., 2002a,b) and persist in the presence of inhibitory RGD peptides (our unpublished data). Mena localized in two distinct patterns within these homophilic adhesions. Fine bands of Mena were seen at the outer margins of cadherin-based lamellipodia, which often, but not invariably, overlapped small clusters and peripheral streaks of E-cadherin staining (Figure 4A). Strikingly, Mena also precisely colocalized with E-cadherin in the macroclusters. It should be noted that many small cadherin clusters did not display detectable Mena staining. This suggested that Mena was specifically recruited to particular regions even within individual adhesive contacts, reminiscent of our observation that Mena localized selectively to subregions within cell–cell contacts (Figure 1).
We then used time-lapse digital videomicroscopy to further compare these two distinct patterns of Mena as hE-CHO cells transiently expressing EGFP-Mena adhered to hE/Fc-coated substrata (Figure 4B and Supplemental Movie 1). Consistent with previous findings (Verma et al., 2004), the outer margins of cadherin-based lamellipodia were very dynamic structures that displayed periodic extension and retraction. EGFP-Mena at the outer margin strictly localized to regions undergoing protrusion, being promptly lost when the margins began to retract. In contrast, EGFP-Mena in macroclusters displayed less dynamic lateral movement, typically persisting in place for the duration of the movies, although some remodeling was observed. Thus, very different motility patterns distinguished these two pools of Mena that coexisted within individual homophilic adhesions. These two pools of Mena also costained with distinct Ena/VASP binding proteins. IRSp53 (Krugmann et al., 2001) colocalized precisely with Mena at the outer margins of cadherin-based lamellipodia but was not detected in Mena macroclusters (Supplemental Figure 3A). In contrast, vinculin colocalized consistently with Mena in macroclusters, but it was more intermittently found at the outer margins of lamellipodia (Supplemental Figure 3B). Together, these findings suggest that within individual cadherin homophilic adhesions Mena is recruited into two distinct cortical pools, which differ in subcellular location, dynamic morphology, and localization with potential associated proteins.
Ena/VASP Activity Supports Distinct Modes of Actin Organization at Cadherin Homophilic Adhesions
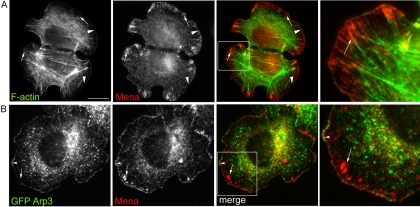
The two pools of Mena identified within individual homophilic cadherin adhesions also coincided with two distinct patterns of F-actin organization (Figure 5A). Prominent bands of F-actin characteristically demarcated the outer margins of cadherin-based lamellipodia, whereas the contacts were traversed by dense actin bundles that terminated in cadherin macroclusters (our unpublished data; Shewan et al., 2005). Mena at the outer margins of lamellipodia colocalized exactly with the broad peripheral bands of F-actin (Figure 5A) and also with transiently expressed GFP-Arp3 (Figure 5B), consistent with our recent observation that this pool of actin reflects Arp2/3-mediated actin nucleation (Verma et al., 2004). Because Arp2/3 activity drives lamellipodial protrusion in cadherin planar adhesion assays (Verma et al., 2004), we refer to this pool of Mena as a “protrusive” pool. In contrast, the pool of Mena that accumulated in cadherin macroclusters formed termini for the actin bundles (Figure 5A). This second (macrocluster) pattern resembled the Mena-enriched puncta found at the termini of perijunctional actin bundles in epithelial monolayers (Figure 1B). These observations suggested that Mena might be common to different modes of actin organization within individual homophilic adhesions.
Figure 5.
Spatial relationships between Mena, F-actin, and Arp2/3 at cadherin homophilic adhesions. (A) hE-CHO cells adherent to hE/Fc-coated substrata were costained for Mena and F-actin. Linear Mena staining colocalized with the thick rim of actin seen at the outer margins of the adhesive zone (arrowheads). Additionally, Mena in macroclusters formed termini for prominent actin bundles that traversed the cells (arrows). A magnified view of the boxed region is shown in the right-hand panel. (B) hE-CHO cells were transiently transfected with EGFP-Arp3 and allowed to adhere to hE/Fc-coated substrata for 90 min. Mena and EGFP-Arp3 coaccumulated with Mena at the outer margins (arrowhead), but EGFP-Arp3 was not observed in Mena-enriched macroclusters (arrows). Bar (A), 10 μm.
We then asked whether Ena/VASP activity was necessary for these two forms of actin organization to be expressed. We tested this using murine fibroblastic MVD7 cells that lack Mena, VASP, and EVL (Bear et al., 2000; Loureiro et al., 2002) and MVD7 cells complemented with EGFP-tagged full-length Mena (MV-FLM cells). Although MVD7 cells possess low levels of endogenous N-cadherin (our unpublished data), they did not spread well upon hE/Fc-coated substrata. Accordingly, to facilitate analysis, human E-cadherin was transiently expressed in both MVD7 and MV-FLM cells (Figure 6A). MVD7 cells expressing hE-cadherin attached to hE/Fc-coated substrata, but they failed to make broad lamellipodia and actin bundles were scant (Figure 6B). Furthermore, although E-cadherin stained in fine puncta, the prominent streaks and macroclusters seen in MCF-7 and hE-CHO cells were conspicuously absent.
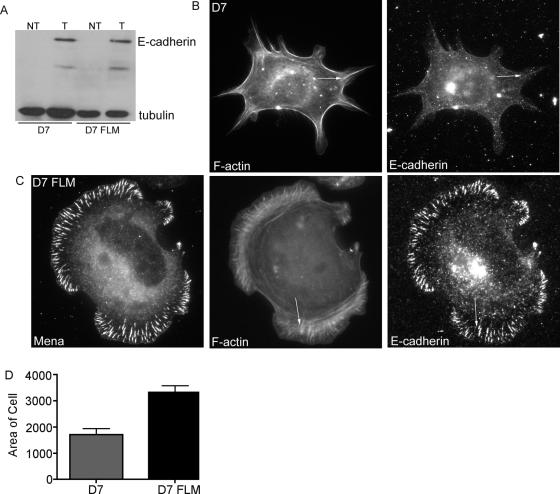
Figure 6.
Mena is necessary and sufficient to support contact zone extension and actin bundling at cadherin homophilic adhesions. MVD7 and MV-FLM cells were transiently transfected with hE-cadherin and allowed to adhere to hE/Fc for 90 min. Cells were triple labeled for E-cadherin, Mena, and F-actin. (A) Western blot analysis confirmed E-cadherin expression in both cell lines. (B) MVD7 cells spread very poorly on hE/Fc and displayed few actin bundles. Fine puncta of E-cadherin formed, however, no prominent macroclusters were detected. In contrast, MV–FLM cells displayed broad lamellipodia and actin bundles which terminated in Mena- and E-cadherin-enriched macroclusters (C, arrow). (D) Adhesive contact areas in MVD7 and MV-FLM cells transiently expressing hE-cadherin were quantitated by measurement of pixel number. Data are means ± SE (n = 20). Bar (B), 10 μm.
In contrast, MV-FLM cells expressing hE-cadherin spread much more extensively on hE/Fc-coated substrata and displayed Mena both at the outer margins of protrusions and in macroclusters (Figure 6C), similar to the patterns of endogenous Mena seen in MCF-7 cells and hE-CHO cells. Mena-complemented cells also generated cadherin-based lamellipodia with prominent peripheral F-actin bands (Figure 6C) that were much broader than those seen in MVD7 cells, an observation that was confirmed by quantitation of adhesive surface area (Figure 6D). Furthermore, actin bundles were restored in Mena-complemented cells. Interestingly, cellular E-cadherin also reaccumulated to form macroclusters that colocalized precisely with EGFP-Mena, as seen with MCF-7 and hE-CHO cells. Together, these observations indicated that Ena/VASP activity supported both the peripheral actin bands and actin bundles found at homophilic adhesive contacts.
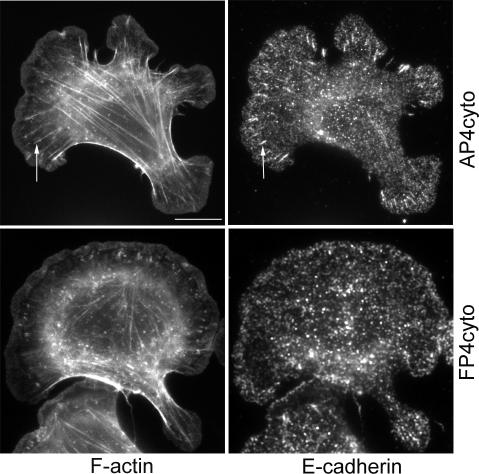
We also asked whether actin organization might be affected by selective displacement of Mena from one of the pools found at homophilic adhesions. We used a cytosolic form of FP4 (FP4-cyto) that displaces Mena from focal adhesions but not from leading edges in migrating cells (Bear et al., 2000). Similarly, in hE/Fc planar adhesion assays FP4-cyto depleted Mena staining in macroclusters but did not affect the protrusive pool of Mena (our unpublished data). In contrast, Mena staining in cells expressing a cytosolic form of AP4 (AP4-cyto) was indistinguishable from mock-transfected controls. MCF7 cells expressing either construct displayed prominent peripheral bands of F-actin upon attachment to hE/Fc-coated substrata (Figure 7). However, cadherin-based actin bundles were much less evident in cells expressing FP4-cyto compared with cells expressing AP4-cyto. This suggested that the selective presence of Mena in the macrocluster pool influenced significantly the assembly of actin filament bundles at cadherin homophilic adhesions, but it was less critical for F-actin organization in cadherin-based protrusions.
Figure 7.
Selective displacement of Ena/VASP proteins from the macrocluster pool ablates cadherin-based actin bundles. hE-CHO cells transiently expressing either FP4-cyto or AP4-cyto were allowed to adhere to hE/Fc-coated substrata. Actin cables (arrows) that terminated in cadherin macroclusters (arrows) were seen in cells expressing AP4-cyto were much less evident in cells expressing FP4-cyto. Bar, 10 μm.
Actin Filament Barbed Ends Are Necessary for the Protrusive Pool of Mena
Multiple mechanisms can determine the subcellular localization of Ena/VASP proteins, including the free barbed ends of actin filaments themselves. To pursue the molecular requirements that target Mena to different regions within individual cadherin adhesive contacts, we first tested the impact of filament barbed ends on Mena recruitment (Figure 8).
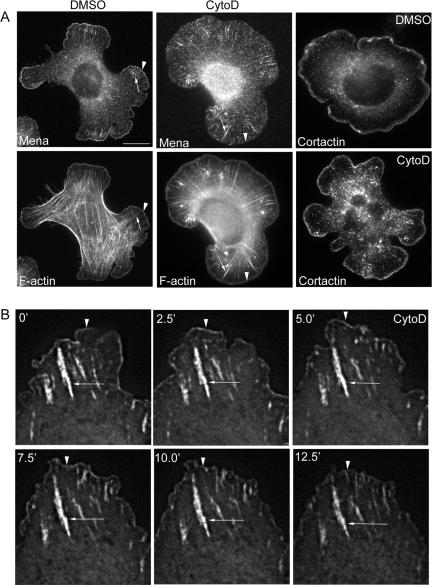
Figure 8.
Effect of CytoD on Mena localization at cadherin adhesions. (A) Cells were plated onto hE/Fc and allowed to adhere for 60 min before being exposed to CytoD (25 nM) or DMSO carrier for a further 30 min. Cells were then fixed and stained for Mena and F-actin or cortactin. Mena localization at the outer margins was substantially reduced by CytoD (arrowhead), but staining in macroclusters was unaffected (arrow). Cortactin localization at cell peripheries was not affected by CytoD. (B) Cells transiently expressing EGFP-Mena were allowed to adhere to hE/Fc-coated substrata and imaged by time-lapse digital fluorescence microscopy. CytoD (25 nM) was added at the indicated time. EGFP-Mena at the peripheries of protrusions (arrowheads) was less prominent after ∼5 min, whereas Mena localization in macroclusters (arrows) persisted. The full movie is shown as Supplemental Movie 2. Bar (A), 10 μm.
Used at low concentrations, cytochalasin D (CytoD) caps the barbed ends of actin filaments and displaces Mena from the tips of fibroblast lamellipodia and growth cone filopodia (Cooper, 1987; Bear et al., 2002). Accordingly, cells were allowed to adhere to hE/Fc-coated substrata and then treated with CytoD in a range of low concentrations (10–50 nM) for a further 30 min before being immunostained for endogenous Mena (our unpublished data). We found that 25–50 nM CytoD effectively displaced Mena from the protrusive pool at the leading edges of cadherin-based lamellipodia, but Mena staining in macroclusters was not disrupted by any of the concentrations used (Figure 8A illustrates data using 25 nM CytoD). Time-lapse movies of cells expressing EGFP-Mena revealed that CytoD (25 nM) decreased EGFP-Mena at protrusive leading edges within ∼5 min of applying the drug to the incubation chamber (Figure 8B and Supplemental Movie 2), but localization in macroclusters persisted. This implied that intact barbed ends of actin filaments were more important to support the protrusive pool of Mena than to support the macrocluster pool. In contrast, cortactin (Helwani et al., 2004) staining at the peripheral margins was not affected by CytoD in these concentrations (Figure 8A). Therefore, not all actin-binding proteins were delocalized by CytoD in these experiments, implying that capping barbed ends might exert a relatively selective effect on Mena localization in the protrusive pool.
Mena Incorporation into Macroclusters Requires Rho Kinase Signaling
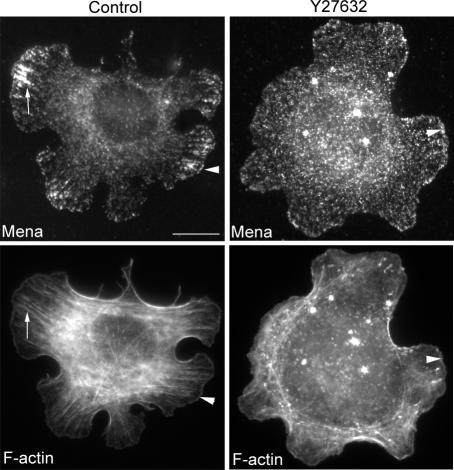
Finally, we sought to identify factors that might influence the localization of Mena in the macrocluster pool. Given the apparent relationship between Mena macroclusters and actin cables, we focused on Rho kinase signaling, which we recently found influences cadherin-based actin bundling (Shewan et al., 2005). To test the impact of Rho kinase on Mena localization at homophilic adhesions, we allowed cells to first adhere and spread on hE/Fc and then treated them briefly with the Rho kinase inhibitor Y27632 in a range of concentrations (1–50 μM). Clear changes were seen using 10 μM Y27632, a concentration that we previously found to perturb cadherin-based actin bundles (Shewan et al., 2005). Mena staining in macroclusters was no longer identifiable, being replaced by finer punctate staining that was more diffusely distributed throughout the adhesive contacts (Figure 9). However, peripheral staining at the leading edges of cadherin-based protrusions was still detectable at all the drug concentrations used. This suggested that the macrocluster pool of Mena was more sensitive to Rho kinase inhibition than was the protrusive pool.
Figure 9.
Rho kinase signaling is necessary for the macrocluster pool of Mena at cadherin homophilic adhesions. Cells were plated onto hE/Fc for 60 min and then treated with Y27632 (10 μM) for a further 30 min. Y27632 disrupted the prominent accumulation of Mena in macroclusters, but linear staining of Mena persisted at the outer margins of cells (arrowhead). Bar, 10 μm.
DISCUSSION
It has long been recognized that classical cadherins cooperate closely with the actin cytoskeleton to assemble and remodel cell–cell contacts (Adams et al., 1996; Adams and Nelson, 1998; Bershadsky, 2004). Recent developments indicate that such cooperation involves more actin regulatory proteins than previously appreciated (Kovacs et al., 2002b; Helwani et al., 2004; Kobielak et al., 2004) and also multiple potentially distinct modes of cytoskeletal activity (Vaezi et al., 2002; Verma et al., 2004). In this report, we focused on analyzing the cellular contribution of Ena/VASP proteins to actin regulation at cadherin contacts. Our interest in this highly conserved family of cytoskeletal regulators was prompted both by their ability to participate in many different actin-dependent cellular processes as well as previous evidence that they localize to cell–cell contacts. Indeed, genetic studies in Drosophila indicate that ena participates in a signal-regulated pathway to control the regional distribution of F-actin within epithelial cells (Grevengoed et al., 2001).
Our findings extend these previous studies to demonstrate that Ena/VASP activity critically influences the actin cytoskeleton at established cell–cell contacts between mammalian cells. Thus, sequestration of Ena/VASP proteins using the FP4-mito strategy reduced the density of actin filaments at MCF-7 cell–cell contacts by ∼50%. This effect on the perijunctional actin cytoskeleton is likely to reflect a major impact of Ena/VASP proteins on processes of actin assembly, because Ena/VASP sequestration also significantly reduced the local incorporation of fluorescent G-actin at contacts. This is consistent with the well documented capacity for Ena/VASP proteins to modulate actin assembly (Skoble et al., 2001), notably by acting at postnucleation steps to control filament length (Bear et al., 2002). However, potential contributions of Ena/VASP activity to other processes, such as filament bundling (Svitkina et al., 2003), are not excluded. It should also be noted that although we principally followed Mena in our experiments, both VASP and EVL can also be found at cell–cell contacts (Vasioukhin et al., 2000; Scott, unpublished data) and are sequestered to mitochondria by FP4-mito (Bear et al., 2000; Scott, unpublished data). Given the high degree of functional redundancy among mammalian Ena/VASP proteins, our findings then define an important requirement for Ena/VASP activity to regulate the actin cytoskeleton at cadherin contacts without necessarily identifying specific contributions of individual members of this protein family.
How, then, are Ena/VASP proteins recruited to regulate the actin cytoskeleton at cadherin contacts? We suggest that this reflects a critical influence of E-cadherin adhesion itself. This possibility was first prompted by the observation that Mena accumulated more prominently at contacts between cadherin-expressing cells than between cadherin-deficient cells, implying that expression of the cadherin could influence the subcellular localization of Mena. This notion was reinforced by the demonstration that binding of cells to immobilized cadherin ligands recruited Mena into sites of adhesion, both when ligands were presented on beads and also as planar adhesive substrata. This indicated that homophilic ligation of E-cadherin receptors was sufficient to recruit Mena to the cell surface independently of other juxtacrine signals that are likely to exist at native cell–cell contacts. By implication, the junctional localization of Ena/VASP proteins at cell–cell contacts may reflect the action of cadherin adhesion itself, perhaps acting through signaling pathways triggered by homophilic ligation (Yap and Kovacs, 2003).
The ability of homophilic ligation to recruit Mena to the cortex gave us the opportunity to explore in greater detail how Mena may distribute within individual adhesive contacts. Strikingly, we found that Mena localized in two clear and distinct patterns when cells adhered to hE/Fc-coated substrata: at the very margins of cadherin-based protrusions as they were extending as well as in large clusters that precisely colocalized with macroclusters of cellular E-cadherin. In addition to their subcellular localization, these pools differed in their motile behavior and in their colocalization with different potential Mena binding proteins. Furthermore, the protrusive pool of Mena seemed to preferentially require intact barbed ends of actin filaments, because Mena was readily displaced from the protrusive pool but not the macrocluster pool by low concentrations of CytoD. This is consistent with evidence from other cellular and in vitro systems that Ena/VASP proteins can interact with uncapped actin filament barbed ends (Barzik et al., 2005). In contrast, inhibition of Rho kinase signaling more readily perturbed Mena localization in macroclusters than its localization in the peripheral pool. Together, these findings indicate that Mena can recruit into two distinct pools within homophilic cadherin adhesions.
Interestingly, the locations of these two pools of Mena also corresponded to morphologically distinct patterns of F-actin at homophilic adhesions. The protrusive pool of Mena colocalized with broad peripheral bands of F-actin, whereas the macrocluster pool localized at the termini of prominent actin bundles. Several recent observations indicate that these patterns correspond to two pools of F-actin that are distinct and experimentally separable. Thus, the protrusive pool seems to involve Arp2/3-driven actin assembly: the Arp2/3 complex specifically localizes in this region of homophilic adhesions, and the peripheral bands of F-actin are lost when Arp2/3 activity is inhibited (Kovacs et al., 2002b; Verma et al., 2004). Indeed, in our present study, we found that Mena in the protrusive pool colocalized with GFP-Arp3. Moreover, the ability of cells to extend protrusions when they adhere to cadherin-coated substrata is severely compromised when Arp2/3 is inhibited, indicating a critical contribution of Arp2/3 to forming these protrusions (Verma et al., 2004). In contrast, Arp2/3 was not seen associated with the cadherin-based actin cables. Instead, Myosin 2 is necessary for the actin bundles but not for the peripheral pool of actin filaments, suggesting a preferential role of actomyosin contractility in generating these cadherin-based actin bundles (Shewan et al., 2005). These findings then suggest that Mena may be common to distinct pools of F-actin found at cadherin homophilic adhesions.
Importantly, Ena/VASP activity played a critical role in the expression of both these modes of actin organization. Thus, Ena/VASP-deficient MVD7 cells expressing E-cadherin adhered to hE/Fc-coated substrata but failed to assemble cadherin-based actin cables, nor did they display the broad lamellipodial protrusions that characterize Arp2/3-driven actin in this assay system. Expression of Mena restored both cadherin-based lamellipodia and cables, indicating that Mena activity alone could support both Arp2/3-dependent cell protrusion and actin bundle assembly in this assay system. These observations are consistent with evidence in other systems that Ena/VASP proteins can affect biochemically distinct modes of actin regulation. For example, Ena/VASP proteins influence both the length of Arp2/3-nucleated filaments at the leading edges of motile cells (Bear et al., 2002) as well as participate in generating parallel actin bundles (Svitkina et al., 2003). Similarly, ectopic accumulation of Ena in abelson-mutant Drosophila embryos caused the mislocalization of both Arp2/3 and the formin Diaphanous, actin nucleators associated with different modes of filament organization (Grevengoed et al., 2003). Together, these findings indicate that Ena/VASP activity can critically regulate two potentially distinct modes of actin organization made by cells at cadherin homophilic adhesions.
Several observations further suggest that these two modes of actin organization may also exist at cell–cell contacts. Thus, actin cables are well-described in the perijunctional regions of cell–cell contacts. During the biogenesis of contacts, these bundles often seem to terminate at nascent adherens junctions (Yonemura et al., 1995), suggesting that they may be anchored to cadherin adhesions. We postulate that the cadherin-based actin bundles found in homophilic adhesion assays may correspond to these perijunctional actin cables. Consistent with this notion, we recently found that inhibition of Myosin 2 perturbed actin bundles both in homophilic adhesion assays and at native cell–cell contacts (Shewan et al., 2005). In terms of the protrusive F-actin pool, the Arp2/3 nucleator that is responsible for generating these filaments is also found at cadherin-based cell–cell contacts, where it contributes to actin assembly (Verma et al., 2004). Together, these observations imply that the capacity of Ena/VASP proteins to modulate both Arp2/3-generated actin structures and actin cables may also exist at native cell–cell contacts. Thus, the observed impact of Ena/VASP proteins on the perijunctional actin cytoskeleton may arise from contributions to distinct modes of actin regulation at those contacts.
It is important to note, however, that in our experiments Ena/VASP activity exerted an important but not essential influence on cytoskeletal regulation by cadherin. Actin assembly and bundle formation persisted, albeit to a reduced extent, despite loss of Ena/VASP function. This is consistent with genetic evidence that UNC-34, the sole Ena/VASP gene in Caenorhabditis elegans, is not essential during development (Withee et al., 2004) as well as with cellular and biochemical evidence that these proteins act in concert with other mechanistically distinct molecules to regulate actin filament dynamics and organization (Krause et al., 2003). It is likely, then, that the impact of Ena/VASP proteins on cadherin–actin cooperation will be influenced by cellular context. Indeed, our observation that Mena localizes most prominently in newly forming contacts suggests that these proteins may exert their action preferentially at dynamic contacts that are forming or remodeling.
In conclusion, our findings demonstrate the capacity of Ena/VASP proteins to regulate the actin cytoskeleton at cadherin-based adhesive contacts. These proteins contribute in significant part by forming part of an ensemble of distinct actin regulators that are recruited to contacts as a direct or indirect response to cadherin adhesion itself. We further postulate that Ena/VASP protein activity may be common to, and important for, the expression of distinct modes of actin organization at cadherin contacts. An interesting question is whether differential regulation of Ena/VASP activity may control the balance between expression of Arp2/3-driven protrusions and actin bundling at contacts. Although our data do not speak directly to this issue, our observation that selective depletion of Ena/VASP proteins from macroclusters affected actin cables more than the protrusive actin bands suggests that Ena/VASP proteins might be able to act semi-independently in these two actin pools. Thus, Ena/VASP proteins may act as higher-order regulators of the actin cytoskeleton at cadherin cell–cell adhesions.
Supplementary Material
Acknowledgments
We thank the aforementioned colleagues who generously provided reagents and all the members of our laboratory for unfailing encouragement, friendship, and support, which we cherish. The research in Australia was funded by the Australian Research Council, J.A.S. was supported by an Australian Postgraduate Award, and A.S.Y. was a Wellcome Trust International Senior Research Fellow.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–07–0644) on December 21, 2005.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
Footnotes
Gavard et al. (2004) describe these structures as “cadherin adhesions” by analogy with integrin-based focal adhesions. We prefer to use the less elegant term, “cadherin macroclusters,” because the smaller cadherin clusters may also mediate adhesion.
References
- Adams, C. L., and Nelson, W. J. (1998). Cytomechanics of cadherin-mediated cell–cell adhesion. Curr. Opin. Cell Biol. 10, 572–577. [DOI] [PubMed] [Google Scholar]
- Adams, C. L., Nelson, W. J., and Smith, S. J. (1996). Quantitative analysis of cadherin-catenin-actin reorganization during development of cell–cell adhesion. J. Cell Biol. 135, 1899–1911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barzik, M., Kotova, T. I., Higgs, H. N., Hazelwood, L., Hanein, D., Gertler, F. B., and Schafer, D. A. (2005). Ena/VASP proteins enhance actin polymerization in the presence of barbed end capping proteins. J. Biol. Chem. 280, 28653–28662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baum, B., and Perrimon, N. (2001). Spatial control of the actin cytoskeleton in Drosophila epithelial cells. Nat. Cell Biol. 3, 883–890. [DOI] [PubMed] [Google Scholar]
- Bear, J. E., Loureiro, J. J., Libova, I., Fassler, R., Wehland, J., and Gertler, F. B. (2000). Negative regulation of fibroblast motility by Ena/VASP proteins. Cell 101, 717–728. [DOI] [PubMed] [Google Scholar]
- Bear, J. E., et al. (2002). Antagonism between Ena/VASP proteins and actin filament capping regulates fibroblast motility. Cell 109, 509–521. [DOI] [PubMed] [Google Scholar]
- Bershadsky, A. (2004). Magic touch: how does cell–cell adhesion trigger actin assembly? Trends Cell Biol. 14, 589–593. [DOI] [PubMed] [Google Scholar]
- Brieher, W. M., and Gumbiner, B. M. (1994). Regulation of C-cadherin function during activin induced morphogenesis of Xenopus animal caps. J. Cell Biol. 126, 519–527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper, J. A. (1987). Effects of cytochalasin and phalloidin on actin. J. Cell Biol. 105, 1473–1478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danjo, Y., and Gipson, I. K. (1998). Actin `purse string' filaments are anchored by E-cadherin-mediated adherens junctions at the leading edge of the epithelial wound, providing coordinated cell movement. J. Cell Sci. 111, 3323–3332. [DOI] [PubMed] [Google Scholar]
- Gavard, J., Lambert, M., Grosheva, I., Marthiens, V., Irinopoulou, T., Riou, J. F., Bershadsky, A., and Mege, R. M. (2004). Lamellipodium extension and cadherin adhesion: two cell responses to cadherin activation relying on distinct signalling pathways. J. Cell Sci. 117, 257–270. [DOI] [PubMed] [Google Scholar]
- Gertler, F. B., Comer, A. R., Juang, J. L., Ahern, S. M., Clark, M. J., Liebl, E. C., and Hoffmann, F. M. (1995). enabled, a dosage-sensitive suppressor of mutations in the Drosophila Abl tyrosine kinase, encodes an Abl substrate with SH3 domain-binding properties. Genes Dev. 9, 521–533. [DOI] [PubMed] [Google Scholar]
- Gertler, F. B., Doctor, J. S., and Hoffmann, F. M. (1990). Genetic suppression of mutations in the Drosophila abl proto-oncogene homolog. Science 248, 857–860. [DOI] [PubMed] [Google Scholar]
- Gertler, F. B., Niebuhr, K., Reinhard, M., Wehland, J., and Soriano, P. (1996). Mena, a relative of VASP and Drosophila Enabled, is implicated in the control of microfilament dynamics. Cell 87, 227–239. [DOI] [PubMed] [Google Scholar]
- Goh, K. L., Cai, L., Cepko, C. L., and Gertler, F. B. (2002). Ena/VASP proteins regulate cortical neuronal positioning. Curr. Biol. 12, 565–569. [DOI] [PubMed] [Google Scholar]
- Grevengoed, E. E., Fox, D. T., Gates, J., and Peifer, M. (2003). Balancing different types of actin polymerization at distinct sites: roles for Abelson kinase and Enabled. J. Cell Biol. 163, 1267–1279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grevengoed, E. E., Loureiro, J. J., Jesse, T. L., and Peifer, M. (2001). Abelson kinase regulates epithelial morphogenesis in Drosophila. J. Cell Biol. 155, 1185–1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gumbiner, B. M. (1996). Cell adhesion: the molecular basis of tissue architecture and morphogenesis. Cell 84, 345–357. [DOI] [PubMed] [Google Scholar]
- Hara, T., Ishida, H., Raziuddin, R., Dorkhom, S., Kamijo, K., and Miki, T. (2004). Novel kelch-like protein, KLEIP, is involved in actin assembly at cell–cell contact sites of Madin-Darby canine kidney cells. Mol. Biol. Cell 15, 1172–1184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helwani, F. M., Kovacs, E. M., Paterson, A. D., Verma, S., Ali, R. G., Fanning, A. S., Weed, S. A., and Yap, A. S. (2004). Cortactin is necessary for E-cadherin-mediated contact formation and actin reorganization. J. Cell Biol. 164, 899–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobielak, A., Pasolli, H. A., and Fuchs, E. (2004). Mammalian formin-1 participates in adherens junctions and polymerization of linear actin cables. Nat. Cell Biol. 6, 21–30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kovacs, E. M., Ali, R. G., McCormack, A. J., and Yap, A. S. (2002a). E-cadherin homophilic ligation directly signals through Rac and phosphatidylinositol 3-kinase to regulate adhesive contacts. J. Biol. Chem. 277, 6708–6718. [DOI] [PubMed] [Google Scholar]
- Kovacs, E. M., Goodwin, M., Ali, R. G., Paterson, A. D., and Yap, A. S. (2002b). Cadherin-directed actin assembly: E-cadherin physically associates with the Arp2/3 complex to direct actin assembly in nascent adhesive contacts. Curr. Biol. 12, 379–382. [DOI] [PubMed] [Google Scholar]
- Krause, M., Dent, E. W., Bear, J. E., Loureiro, J. J., and Gertler, F. B. (2003). Ena/VASP proteins: regulators of the actin cytoskeleton and cell migration. Annu. Rev. Cell Dev. Biol. 19, 541–564. [DOI] [PubMed] [Google Scholar]
- Krugmann, S., Jordens, I., Gevaert, K., Driessens, M., Vandekerckhove, J., and Hall, A. (2001). Cdc42 induces filopodia by promoting the formation of an IRSp 53, Mena complex. Curr. Biol. 11, 1645–1655. [DOI] [PubMed] [Google Scholar]
- Lanier, L. M., Gates, M. A., Witke, W., Menzies, A. S., Wehman, A. M., Macklis, J. D., Kwiatkowski, D., Soriano, P., and Gertler, F. B. (1999). Mena is required for neurulation and commissure formation. Neuron 22, 313–325. [DOI] [PubMed] [Google Scholar]
- Lebrand, C., Dent, E. W., Strasser, G. A., Lanier, L. M., Krause, M., Svitkina, T. M., Borisy, G. G., and Gertler, F. B. (2004). Critical role of Ena/VASP proteins for filopodia formation in neurons and in function downstream of netrin-1. Neuron 42, 37–49. [DOI] [PubMed] [Google Scholar]
- Loureiro, J. J., Rubinson, D. A., Bear, J. E., Baltus, G. A., Kwiatkowski, A. V., and Gertler, F. B. (2002). Critical roles of phosphorylation and actin binding motifs, but not the central proline-rich region, for Ena/vasodilator-stimulated phosphoprotein (VASP) function during cell migration. Mol. Biol. Cell 13, 2533–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miki, H., Yamaguchi, H., Suetsugu, S., and Takenawa, T. (2000). IRSp53 is an essential intermediate between Rac and WAVE in the regulation of membrane ruffling. Nature 408, 732–735. [DOI] [PubMed] [Google Scholar]
- Nagafuchi, A., Takeichi, M., and Tsukita, S. (1991). The 102 kd cadherin-associated protein: similarity to vinculin and posttranscriptional regulation of expression. Cell 65, 849–857. [DOI] [PubMed] [Google Scholar]
- Paterson, A. D., Parton, R. G., Ferguson, C., Stow, J. L., and Yap, A. S. (2003). Characterization of E-cadherin endocytosis in isolated MCF-7 and chinese hamster ovary cells: the initial fate of unbound E-cadherin. J. Biol. Chem. 278, 21050–21057. [DOI] [PubMed] [Google Scholar]
- Pollard, T. D., and Borisy, G. G. (2003). Cellular motility driven by assembly and disassembly of actin filaments. Cell 112, 453–465. [DOI] [PubMed] [Google Scholar]
- Reinhard, M., Halbrugge, M., Scheer, U., Wiegand, C., Jockusch, B. M., and Walter, U. (1992). The 46/50 kDa phosphoprotein VASP purified from human platelets is a novel protein associated with actin filaments and focal contacts. EMBO J. 11, 2063–2070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rimm, D. L., Koslov, E. R., Kebriaei, P., Cianci, C. D., and Morrow, J. S. (1995). Alpha 1(E)-catenin is an actin-binding and -bundling protein mediating the attachment of F-actin to the membrane adhesion complex. Proc. Natl. Acad. Sci. USA 92, 8813–8817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottner, K., Behrendt, B., Small, J. V., and Wehland, J. (1999). VASP dynamics during lamellipodia protrusion. Nat. Cell Biol. 1, 321–322. [DOI] [PubMed] [Google Scholar]
- Schafer, D. A., Welch, M. D., Machesky, L. M., Bridgman, P. C., Meyer, S. M., and Cooper, J. A. (1998). Visualization and molecular analysis of actin assembly in living cells. J. Cell Biol. 143, 1919–1930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shewan, A. M., Maddugoda, M., Kraemer, A., Stehbens, S. J., Verma, S., Kovacs, E. M., and Yap, A. S. (2005). Myosin 2 is a key Rho kinase target necessary for the local concentration of E-cadherin at cell–cell contacts. Mol. Biol. Cell 16, 4531–4532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoble, J., Auerbuch, V., Goley, E. D., Welch, M. D., and Portnoy, D. A. (2001). Pivotal role of VASP in Arp2/3 complex-mediated actin nucleation, actin branch-formation, and Listeria monocytogenes motility. J. Cell Biol. 155, 89–100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svitkina, T. M., Bulanova, E. A., Chaga, O. Y., Vignjevic, D. M., Kojima, S., Vasiliev, J. M., and Borisy, G. G. (2003). Mechanism of filopodia initiation by reorganization of a dendritic network. J. Cell Biol. 160, 409–421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeichi, M. (1991). Cadherin cell adhesion receptors as a morphogenetic regulator. Science 251, 1451–1455. [DOI] [PubMed] [Google Scholar]
- Tepass, U., Truong, K., Godt, D., Ikura, M., and Peifer, M. (2000). Cadherins in embryonic and neural morphogenesis. Nat. Rev. Mol. Cell. Biol. 1, 91–100. [DOI] [PubMed] [Google Scholar]
- Vaezi, A., Bauer, C., Vasioukhin, V., and Fuchs, E. (2002). Actin cable dynamics and Rho/Rock orchestrate a polarized cytoskeletal architecture in the early steps of assembling a stratified epithelium. Dev. Cell 3, 367–381. [DOI] [PubMed] [Google Scholar]
- Vasioukhin, V., Bauer, C., Yin, M., and Fuchs, E. (2000). Directed actin polymerization is the driving force for epithelial cell–cell adhesion. Cell 100, 209–219. [DOI] [PubMed] [Google Scholar]
- Verma, S., Shewan, A. M., Scott, J. A., Helwani, F. M., den Elzen, N. R., Miki, H., Takenawa, T., and Yap, A. S. (2004). Arp2/3 activity is necessary for efficient formation of E-cadherin adhesive contacts. J. Biol. Chem. 279, 34062–34070. [DOI] [PubMed] [Google Scholar]
- Watabe-Uchida, M., Uchida, N., Imamura, Y., Nagafuchi, A., Fujimoto, K., Uemura, T., Vermeulen, S., van Roy, F., Adamson, E. D., and Takeichi, M. (1998). alpha-Catenin-vinculin interaction functions to organize the apical junctional complex in epithelial cells. J. Cell Biol. 142, 847–857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waterman-Storer, C. M., Salmon, W. C., and Salmon, E. D. (2000). Feedback interactions between cell–cell adherens junctions and cytoskeletal dynamics in newt lung epithelial cells. Mol. Biol. Cell 11, 2471–2483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Withee, J., Galligan, B., Hawkins, N., and Garriga, G. (2004). Caenorhabditis elegans WASP and Ena/VASP proteins play compensatory roles in morphogenesis and neuronal cell migration. Genetics 167, 1165–1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yap, A. S., Brieher, W. M., and Gumbiner, B. M. (1997). Molecular and functional analysis of cadherin-based adherens junctions. Annu. Rev. Cell Dev. Biol. 13, 119–146. [DOI] [PubMed] [Google Scholar]
- Yap, A. S., and Kovacs, E. M. (2003). Direct cadherin-activated cell signaling: a view from the plasma membrane. J. Cell Biol. 160, 11–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yonemura, S., Itoh, M., Nagafuchi, A., and Tsukita, S. (1995). Cell-to-cell adherens junction formation and actin filament organization: similarities and differences between non-polarized fibroblasts and polarized epithelial cells. J. Cell Sci. 108, 127–142. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.