Abstract
Shiga toxin (Stx) is composed of an A-moiety that inhibits protein synthesis after translocation into the cytosol, and a B-moiety that binds to Gb3 at the cell surface and mediates endocytosis of the toxin. After endocytosis, Stx is transported retrogradely to the endoplasmic reticulum, and then the A-fragment enters the cytosol. In this study, we have investigated whether toxin-induced signaling is involved in its entry. Stx was found to activate Syk and induce rapid tyrosine phosphorylation of several proteins, one protein being clathrin heavy chain. Toxin-induced clathrin phosphorylation required Syk activity, and in cells overexpressing Syk, a complex containing clathrin and Syk could be demonstrated. Depletion of Syk by small interfering RNA, expression of a dominant negative Syk mutant (Syk KD), or treatment with the Syk inhibitor piceatannol inhibited not only Stx-induced clathrin phosphorylation but also endocytosis of the toxin. Also, Golgi transport of Stx was inhibited under all these conditions. In conclusion, our data suggest that Stx regulates its entry into target cells.
INTRODUCTION
The bacterial toxin Shiga toxin (Stx) inhibits protein synthesis in cells after first binding to Gb3 at the cell surface. Then, the toxin is endocytosed, and thereafter it is transported retrogradely to the Golgi apparatus and the endoplasmic reticulum (ER) before being translocated to the cytosol where it inactivates the ribosomes enzymatically (for reviews, see Sandvig and van Deurs, 2002; Sandvig et al., 2002). The toxin is composed of two moieties: the enzymatically active A-chain that is noncovalently attached to a stable pentamer of B-chains that binds to Gb3 and mediates the endocytic uptake of the toxin. In some cells, Stx is endocytosed mainly by clathrin-dependent endocytosis (Sandvig, 2001), although other mechanisms exist (Nichols et al., 2001; Lauvrak et al., 2004; Saint-Pol et al., 2004). Importantly, the toxin can induce its own transport to clathrin-coated pits (Sandvig et al., 1989). When Stx is added to HeLa cells at 0°C, the toxin becomes evenly distributed at the cell surface. However, after a short incubation at 37°C, the toxin moves to clathrin-coated pits and is internalized. In toxin-sensitive cells a larger fraction of the toxin seems to be internalized from clathrin-coated pits at the cell surface, and clathrin is required for endosome-to-Golgi transport of the toxin as well (Lauvrak et al., 2004; Saint-Pol et al., 2004). Because it has been shown by different groups that the fatty acid of Gb3 is important for efficient Golgi transport (Sandvig et al., 1994; Lingwood, 1999), the composition of Gb3 also may play a role for the endocytic pathway used. A raft localization of StxB was recently found to be required for efficient retrograde transport (Falguieres et al., 2001).
In some hematopoetic cells, Stx has been shown to trigger a rapid signaling cascade that might lead to apoptosis (for review, see Cherla et al., 2003). In a number of other cell types induction of signaling leading to apoptosis seems to be mediated by the ribocytotoxic stress induced by the A-fragment after entry into the cytosol (Foster and Tesh, 2002; Smith et al., 2003). Thus, in the latter cases it takes time before the reported signaling takes place.
Because Stx seems to affect its own endocytosis by inducing its transport to clathrin-coated pits (Sandvig et al., 1989), we decided to investigate whether this might occur as a response to a rapid toxin-induced signaling, similarly to what has been reported for endocytic uptake of other ligands. The ability of extracellular ligands to trigger signaling cascades has been well characterized (for review, see Hunter, 2000). The most striking effect is a shift in phospho-proteins, mediated by kinases and phosphatases able to modify the phosphorylation status of cellular proteins (Hunter, 2000). Stimulation of cells by, for example, growth factors needs to be restricted to a spatiotemporal window. To this end, cells have developed a battery of processes capable of attenuating the effect of the stimulating ligand. One of these is the endocytosis and subsequent lysosomal degradation of a ligand in complex with its receptor (for review, see Sorkin and Von Zastrow, 2002). This degradation is tightly regulated, and, interestingly, the signaling cascade triggered by the receptor is also regulating the degradation of the ligand. Thus ligands are able to affect their own intracellular trafficking (Di Fiore and De Camilli, 2001; Gonzalez-Gaitan and Stenmark, 2003; Teis and Huber, 2003; Bache et al., 2004). One of the most studied families of endocytosed receptors is the antibody receptors FcR (Daeron, 1997). These receptors do not possess any enzymatic activity, thus they need to recruit kinases to trigger a signaling cascade within the cell and also to regulate their internalization. Src family kinases (SFKs) and Syk kinase seem to be crucial partners in this process (van Oers and Weiss, 1995; Bolen and Brugge, 1997). Sequential activation of SFKs and Syk is necessary for both the uptake of the receptor into the cell (Park and Schreiber, 1995; Majeed et al., 2001; Strzelecka-Kiliszek et al., 2002) and for the trafficking to lysosomes (Bonnerot et al., 1998). Similarly, Stx in human B-cells activates at least two main tyrosine kinases, Lyn (a SFK) and Syk (Mori et al., 2000), and this activation occurs very fast. Also, in ACHN renal cells Stx activates the SFK Yes (Katagiri et al., 1999). In this paper, the authors were able to show that this tyrosine kinase is recruited to Gb3-enriched structures and occurs in the same fraction as Stx in a sucrose gradient.
In the present study, we have asked the following question: Is Stx able to regulate and increase the efficiency of its entry and intracellular transport by such signaling? We have investigated the signaling occurring upon binding of Stx to HeLa cells, a highly toxin-sensitive cell line previously found to internalize Stx at least partly through clathrin-coated pits. In this paper, we provide evidence for the involvement of a tyrosine kinase in uptake and intracellular transport of Stx. As demonstrated, the toxin induces phosphorylation of clathrin by a Syk-dependent pathway that involves the formation of a complex containing both clathrin heavy chain (CHC) and Syk. Depletion of Syk by small interfering RNA (siRNA), overexpression of a kinase dead mutant of Syk (Syk KD), or inhibition of Syk activity by addition of piceatannol or the more general tyrosine kinase inhibitor genistein, all reduced the uptake of Stx, indicating that toxin signaling is important for its entry. Furthermore, toxin-induced tyrosine phosphorylation of CHC is inhibited in cells treated with siRNA against Syk, or overexpressing Syk KD, but increased when Syk wild-type (WT) is overexpressed. Finally, we here show that Golgi transport of Stx also is reduced under all conditions were Syk activity is abolished, reflecting the fact that inhibition of signaling decreases the entry of the toxin.
MATERIALS AND METHODS
Materials
2-Mercaptoethanesulfonic acid, sodium salt (MESNa), n-octyl-glucopyranoside, HEPES, and Na-vanadate were purchased from Sigma-Aldrich (St. Louis, MO). Na235SO4, [γ-33P]ATP and [3H]leucine were from Amersham Biosciences (Little Chalfont, Buckinghamshire, United Kingdom). Rabbit anti-Stx antibody was obtained by standard immunization. Rabbit polyclonal anti-Syk antibody (N-19) and mouse monoclonal anti-Syk (4D10) were from Santa Cruz Biotechnology (Santa Cruz, CA). 4G10 mouse monoclonal anti-phosphotyrosine antibody was from Upstate Biotechnology (Lake Placid, NY). Anti-CHC antibodies are mouse monoclonal X-22 (Abcam, Cambridge, United Kingdom) and mouse monoclonal N-terminal fragment (Research Diagnostics, Flanders, NJ). Genistein (BIOMOL Research laboratories, Plymouth Meeting, PA), piceatannol (Calbiochem, San Diego, CA), and SU6656 (Calbiochem) were resuspended in dimethyl sulfoxide (DMSO), and frozen aliquots were stored at –20°C. When used in experiments, the DMSO concentration never exceeded 0.2%. The StxB plasmid encoding the B-chain-Sulf2 was a kind gift from Dr. B. Goud (Institut Curie, Paris, France).
Cells
HeLa cells were grown under 5% CO2 in DMEM with 10% fetal calf serum (FCS). Both media were supplemented with penicillin at 100,000 U/l, streptomycin at 100,000 U/l, and l-glutamine at 2 mM.
Cloning, Mutagenesis, and Transfection
An expressed sequence tag containing the complete coding sequence of Syk (clone BC011399; Invitrogen, Carlsbad, CA) was amplified by Pfx polymerase (Invitrogen) using specific primers (5′-ATA GAA TTC GCA TGG CCA GCA GCG GCA-3′ and 5′-GCT CTA GAT TAGT TCA CCA CGT CAT-3′). The amplicon was digested with EcoRI and XbaI (underlined) and cloned into a pcDNA4 vector (Invitrogen). Kinase dead mutant (Syk KD) was generated using specific primers designed to change K402 to an arginine (5′-AAA CCG TGG CTG TGA gAA TAC TGA AAA ACG-3′ and 5′-CGT TTT TCA GTA TTc TCA CAG CCA CGG TTT-3′) and the human Syk cDNA as a template, the position changed is shown in lowercase letters. The mutagenesis was performed using the QuikChange kit (Stratagene, La Jolla, CA) following the manufacturer's procedure. The constructs were totally sequenced.
Cells were seeded out into 5-cm dishes (2 × 105 cells/dish) or into a 24-well plate (2 × 104 cells/well) and grown overnight. Then, the cells were transfected with Syk WT or Syk KD using FuGENE-6 (Roche Diagnostics, Indianapolis, IN) according to the manufacturer's manual. The cells were left for 24 h before they were used for experiments.
For siRNA transfection studies, cells were seeded out in the absence of penicillin and streptomycin. After 24 h, the cells were transfected with either SMARTpool Syk siRNA, containing four designed siRNAs against Syk pooled (Upstate Biotechnology) (which we have termed Syk siRNA 1), Syk siRNA 2 (5′-GGAUGCUGGUUAUGGAGAU-3′), or a nonspecific control pool (Upstate Biotechnology) by using Oligofectamine (Invitrogen) according to the procedure given by the company. Four hours after transfection, 10% FCS, penicillin at 100,000 U/l, and streptomycin at 100,000 U/l were added to the cells, and the cells were further left for ∼40 h before they were used for experiments. To verify knockdown of Syk, cells were harvested for real-time reverse transcription-PCR ∼2 d after transfection. RNA was isolated using the TRIzol reagent (Invitrogen), and cDNA was synthesized using the iScriptcDNA synthesis kit from Bio-Rad (Hercules, CA), following the manufacturer's instructions. PCR amplifications were conducted using the iQSYBR Green Supermix (Bio-Rad). The PCR reaction was performed in an iCycler thermal cycler with an iCycler iQ real-time PCR detection system (Bio-Rad) under the following conditions: 3 min at 95°C to activate the enzyme followed by 50 cycles at 95°C denaturation for 10 s and 60°C annealing and extension for 35 s. Finally, a melt-curve analysis was added to exclude nonspecific amplification. The expression of glyceraldehyde-3-phosphate dehydrogenase (GAPDH) mRNA was used as an internal control in all experiments. Primer sets used in the PCR reactions were as follows: for Syk, 5′-TGGAGATGGCAGAACTTGGT-3′ and 5′-TTCTTATCCTTGACATGTCTGT-3′ and for GAPDH, 5′-GGAAGGTGAAGGTCGGAGTC-3′ and 5′-GGAAGATGGTGATGG GATTTC-3′. The amount of Syk mRNA was quantified to be almost completely removed (with 80–90%) in our experiments after 2 d of transfection with 200 nM Syk siRNA 1. Syk siRNA 2 was slightly less efficient; the same time and concentration reduced the Syk mRNA level by 70–80%.
Immunoprecipitation and Immunoblotting
Clathrin was immunoprecipitated from the sonicated 2-(N-morpholino)ethanesulfonic acid (MES)-buffer cell lysates by using the mouse monoclonal clathrin heavy chain antibody (X22) (Abcam) prebound to protein G-Sepharose beads (overnight; 4°C). The immunoprecipitates was washed (3 times) with Tris-buffered saline (20 mM Tris-Cl, pH 7.6, and 0.5 M NaCl) and solubilized in SDS-PAGE sample buffer. The immunoprecipitated clathrin was run on a 7.5% SDS-PAGE, and the proteins were blotted onto a polyvinylidene diflouride (PVDF) membrane. The presence of phosphorylated clathrin was detected using an anti-phosphotyrosine horseradish peroxidase-conjugate (BD Transduction Laboratories, Lexington, KY) and the enhanced signal chemiluminescence reagent (Pierce Chemical, Rockford, IL).
Tyrosine-phosphorylated clathrin was immunoprecipitated by using the slurry of an anti-phosphotyrosine column (Upstate Biotechnology) in batch (Walchli et al., 2004). Briefly, ∼40 μl of the slurry was incubated with 106 cells previously lysed and sonicated in MES buffer (0.1 M MES, pH 6.5, 0.5 mM MgCl2, 1 mM EGTA, 1 mM orthovanadate, and a Complete protease inhibitor cocktail [Roche Diagnostics]) overnight at 4°C. After several washes of the slurry with MES buffer, the tyrosine-phosphorylated proteins were eluted with 65 μl of MES buffer containing phenylphosphate. Then, 20 μl of this eluate was run on a 7% SDS-PAGE. After transfer of proteins onto a PVDF membrane, the presence of clathrin was detected by using a mouse monoclonal antibody (Research Diagnostics).
Syk detection was performed as follows. After transfection, cells were grown in complete medium for 24 h. They were then starved in HEPES medium for 4 h and treated with Stx for the indicated time. If inhibitors were used, the cells were preincubated with these compounds for 30 min before the addition of the toxin. After the incubation times indicated, lysates were prepared in NP-40.buffer (50 mM Tris, pH 7.4, 1% NP-40, 0.25% sodium deoxycholate, 150 mM NaCl, 1 mM EDTA, and a tablet of Complete protease inhibitor cocktail [Roche Diagnostics]). Then, the lysates were passed trough a 21-gauge syringe, centrifuged for 5 min at 5000 rpm in an Eppendorf centrifuge, and sonicated three times for 10 s. The cleared supernatant was used for immunoprecipitation (IP) with rabbit polyclonal anti-Syk antibody (N-19) (Santa Cruz Biotechnology) precoated on protein A/G beads overnight at 4°C. The IP was then washed twice in phosphate-buffered saline (PBS)/NP-40 lysis buffer (1:1), and the adsorbed material was analyzed by 7.5% SDS-PAGE under reducing conditions and transferred onto a PVDF membrane. The presence of Syk kinase was detected using mouse anti-Syk antibody (4D10) (Santa Cruz Biotechnology), and the phosphorylation state of Syk was analyzed using a mouse anti-phosphotyrosine antibody. Coimmunoprecipitation (CoIP) of clathrin was analyzed using a mouse anti-CHC antibody. Signal intensities of the bands were quantified using the ImageQuant 5.0 software (Amersham Biosciences).
Preparation of STxB-Sulf2
The modified version of Shiga toxin B-chain containing a tandem of sulfation sites (STxB-Sulf2) was produced in Escherichia coli BL21 (DE3) cells essentially as described previously (Su et al., 1992). Briefly, a 10-ml overnight bacterial culture grown at 37°C was inoculated in 500 ml of LB medium and grown further to an OD600 of 0.6. The culture was heat induced for 4 h at 42°C, and the cells were harvested by centrifugation. The pellet was washed twice with 10 mM Tris-HCl, pH 8; resuspended in 25% sucrose, 1 mM Na2EDTA, and 10 mM Tris-HCl, pH 8.0; and gently shaken at 30°C for 10 min. Cells were harvested by centrifugation and resuspended in ice-cold distilled water. After centrifugation, the supernatant was dialyzed overnight against 20 mM Tris-HCl, pH 7.5, loaded on a Resource Q column (Amersham Biosciences), and eluted with a 0–600 mM NaCl gradient in 20 mM Tris-HCl, pH 7.5.
Sulfation of STxB-Sulf2
HeLa cells were washed twice with sulfate-free medium (MEM 12–126; Cambrex Bio Science, Verviers, Belgium) before incubation with 0.2 mCi/ml Na235SO4 for 3 h at 37°C in the same medium. When indicated, different inhibitors were added to the medium during the last 30 min of this incubation. Then STxB-Sulf2 was added to the medium, and the incubation was continued for 1 h. The cells were then washed twice with cold PBS before they were lysed (0.1 M NaCl, 10 mM Na2HPO4, pH 7.4, 1 mM EDTA, 1% Triton X-100, and 60 mM n-octyl-glucopyranoside, supplemented with a mixture of Complete protease inhibitors [Roche Diagnsotics]). The lysate was centrifuged for 10 min at 5000 rpm in an Eppendorf centrifuge, and the cleared lysate was immunoprecipitated with rabbit anti-Stx antibody immobilized on protein A-Sepharose beads (Amersham Biosciences) overnight at 4°C. The beads were then washed twice with PBS containing 0.35% Triton X-100, and the adsorbed material was analyzed by 12% SDS-PAGE under reducing conditions and transferred onto a PVDF membrane. The membrane was then exposed to a PhosphoImager screen (Amersham Biosciences), and signal intensities of the bands were quantified using the ImageQuant 5.0 software.
Preparation of TAG- and Biotin-labeled Stx and Transferrin
Stx and transferrin were labeled with N-hydroxysuccinimide ester-activated Tris(bipyridine) chelated ruthenium(II) TAG (BioVeris, Gaithersburg, MD) according to the manufacturer's procedure and simultaneously biotinylated with the reducible ImmunoPure NHS-SS-Biotin (Pierce Chemical).
Endocytosis Assays
Endocytosis of Stx and transferrin was measured using a BioVeris detection system, which uses electrochemiluminescence detection, as described previously (Lauvrak et al., 2004). Briefly, the cells were washed with HEPES medium and incubated with TAG- and biotin-labeled Stx (25 ng/ml) in HEPES medium containing 2 mg/ml bovine serum albumin (BSA) for 20 min at 37°C or with TAG- and biotin-labeled transferrin (50 ng/ml) for 5 min at 37°C. When indicated, the cells were preincubated with different inhibitors for 30 min before incubation with Stx. The cells were subsequently washed twice with cold buffer (0.14 M NaCl, 2 mM CaCl2, and 20 mM HEPES, pH 8.6). Then, to remove the SS-linked biotin on cell surface-bound protein, half of the cells were treated with 0.1 M MESNa in the same buffer but supplemented with 2 mg/ml BSA for 20 min at 0°C. The other half was incubated with BSA-containing buffer alone. Subsequently, the cells were washed twice in cold buffer (0.14 M NaCl, 2 mM CaCl2, and 20 mM HEPES, pH 7.4) before they were lysed (0.1 M NaCl, 5 mM MgCl2, 50 mM HEPES, pH 7.0, 1% Triton X-100, and 60 mM n-octyl-glucopyranoside). The lysates were centrifuged for 5 min at 14,000 rpm in an Eppendorf centrifuge, and the amount of TAG- and biotin-labeled Stx or transferrin in the cleared lysates were measured using streptavidin beads (Dynal Biotech, Oslo, Norway) and the BioVeris detection system instrument. In the cell lysate, only protein that is TAG-labeled and still biotinylated is detected via binding to the streptavidin beads; thus, MESNa-treated cells represent the amount of endocytosed protein, whereas untreated cells represent the total amount of protein associated with the cells. Endocytosis of iodinated ricin and transferrin was monitored as in Rodal et al. (1999).
Immunofluorescence
Cells grown on coverslips were transfected with Syk WT or Syk KD 24 h before the experiment. After two washes with HEPES medium, the cells were incubated with Shiga toxin (250 ng/ml) for 45 min at 37°C. The cells were then wash twice with HEPES medium and incubated at 37°C for the indicated time. The cells were subsequently fixed with 3% paraformaldehyde in PBS, permeabilized with 0.2% Triton X-100 for 7 min, and blocked with 5% FCS. Transfected cells were identified using rabbit anti-Syk antibodies (Santa Cruz Biotechnology) followed by Cy3-labeled donkey anti-rabbit IgG (Jackson ImmunoResearch Laboratories, West Grove, PA), and Stx was revealed using the mouse anti-Stx (VT1; Toxin Technology, Sarasota, FL) and a Cy2-labeled donkey anti-mouse IgG (Jackson ImmunoResearch Laboratories). The fixed cells were analyzed using a LSM 510 Meta confocal microscope (Carl Zeiss, Jena, Germany). Pictures were taken of thin single plane sections.
RESULTS
Stx Induces Tyrosine Phosphorylation of Clathrin
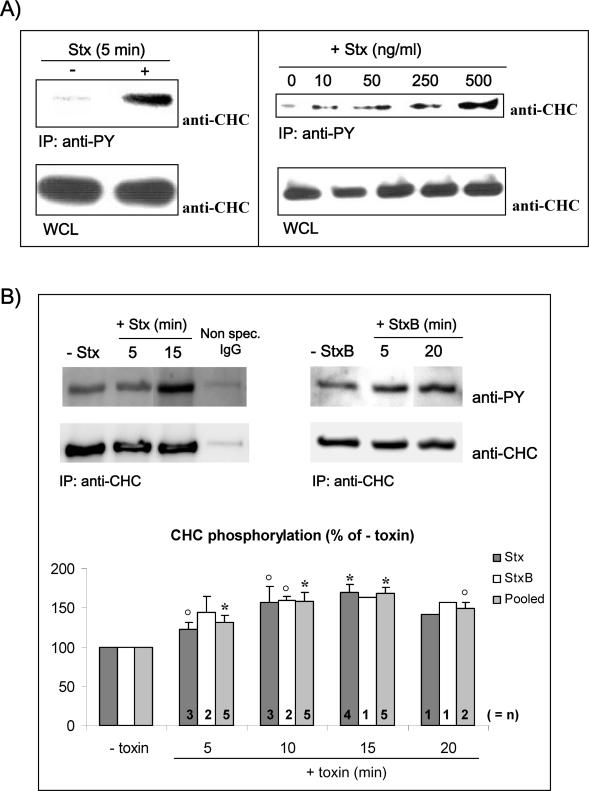
Phosphorylation of CHC has previously been shown to occur upon binding of epidermal growth factor (EGF) (Wilde et al., 1999) and during internalization of the B-cell receptor (BCR) from clathrin-coated pits (Stoddart et al., 2002). Similarly to EGF and the BCR, Stx seems to be internalized from clathrin-coated pits in HeLa cells by a toxin-induced mechanism (Sandvig et al., 1989). We therefore investigated whether Stx, although it binds a glycolipid receptor, can affect clathrin phosphorylation. It has previously been shown that Stx is able to induce a stimulation of tyrosine phosphorylation that peaks after 5 min in lymphocytes (Foster et al., 2000; Mori et al., 2000). Similarly, we found that also in HeLa cells Stx induced tyrosine phosphorylation of a variety of proteins even after 5 min of incubation (our unpublished data). To investigate whether clathrin was one of these phosphorylated proteins, the pool of tyrosine-phosphorylated proteins was immunoprecipitated by using anti-phosphotyrosine (anti-PY) antibodies (see Materials and Methods), and CHC was visualized by Western blot analysis. As shown in Figure 1A (left), CHC rapidly gets phosphorylated upon Stx binding. The amount of clathrin in the whole cell lysates (WCLs) was the same. The Stx-induced CHC phosphorylation was dependent on the amount of toxin added; it increased upon incubation with increasing amounts of Stx (Figure 1A, right). It should be noted that even as little as 10 ng/ml Stx gave a strong increase in CHC phosphorylation. To confirm this Stx-dependent clathrin phosphorylation, we also performed IP of clathrin and then revealed the presence of phosphorylated CHC with anti-PY antibodies (Figure 1B). An increase in phosphorylated clathrin after stimulation with Stx (Figure 1B, left) as well as the B-subunit alone (Figure 1B, right) was also observed by this approach. The figure further shows that CHC is constitutively phosphorylated in HeLa cells. Quantification of the amount of phosphorylated clathrin from 8 experiments (n = 1–5 for the different time points) revealed that there was an increase already after 5 min and that the peak was seen after 15 min with an increase to ∼170% for both Stx and StxB (Figure 1B, graph). From these data, we can conclude that Stx activates a signaling cascade involving tyrosine kinases that increase the phosphorylation status of CHC.
Figure 1.
Tyrosine phosphorylation of clathrin in response to Stx. (A) HeLa cells were incubated with or without Stx (250 ng/ml) for 5 min (left) or with increasing concentrations of Stx for 5 min at 37°C (right). The cells were then lysed, and tyrosine phosphorylated proteins were immunoprecipitated by using the slurry of an anti-PY column. The column elution was analyzed by immunoblotting with mouse anti-CHC. As a control, a fraction of the WCL was run in parallel, and CHC was detected. (B) HeLa cells treated with or without Stx or StxB (250 ng/ml) for the indicated time points were lysed and immunoprecipitated with mouse anti-CHC antibody (X22). As a negative control, cells were also immunoprecipitated with nonspecific mouse IgG. The immunoprecipitates were analyzed by immunoblotting with mouse anti-PY. To check equal loading, the membrane was then stripped and reprobed with anti-CHC. Note that all lanes shown for Stx are from the same blot, and all lanes shown for StxB are from the same blot. Graph, quantification of the amount of phosphorylated CHC (after normalization for the amount of immunoprecipitated CHC) after Stx and StxB treatment, and after pooling these results. The error bars show the SEM (n > 2) or the deviation (n = 2) for the different time points. Significant differences (p < 0.05) where determined by Student's t test and are indicated by a star; 0.05 < p <0.114 are indicated by open circles (○). Together, these p values indicate that the increase in CHC phosphorylation is significant.
Syk Becomes Activated upon Stx Binding
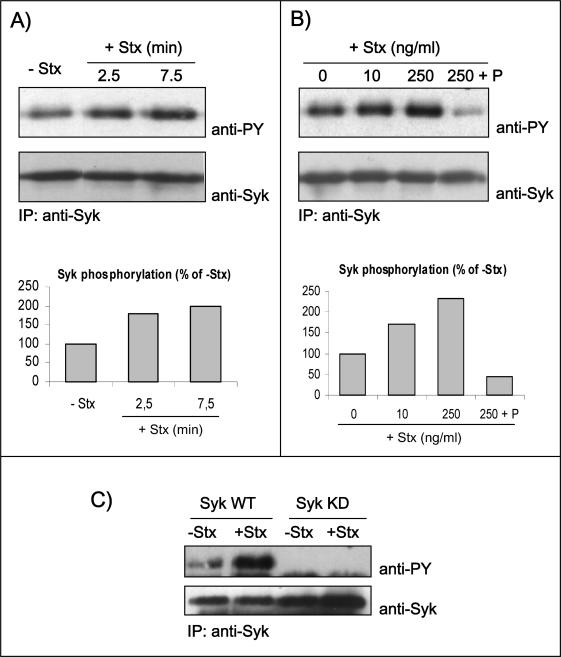
Because the tyrosine kinase Syk is activated by the StxB subunit in B cells (Mori et al., 2000), we decided to investigate whether Syk is activated during Stx entry into HeLa cells. It is known that Syk is present at a low concentration in HeLa cells (Renedo et al., 2001; our unpublished data) as well as in many other nonlymphoid cell lines (Yanagi et al., 2001). To facilitate our studies, we therefore overexpressed human Syk in HeLa cells and monitored toxin-induced tyrosine phosphorylation of Syk after immunoprecipitation of the kinase. Indeed, Syk autophosphorylation is accepted as a hallmark of its activation state (Rowley et al., 1995). As shown in Figure 2A, Stx holotoxin (as well as the purified B-chain; Figure 4) triggers an early signaling reflected by fast phosphorylation of Syk. Quantification revealed that the amount of phosphorylated Syk was increased to 180% already after 2.5 min, and after 7.5 min the amount was doubled compared with nonstimulated cells (Figure 2A, graph). Furthermore, as little as 10 ng/ml toxin is sufficient to activate the kinase (Figure 2B). However, a higher toxin concentration (250 ng/ml) gave a stronger signal, and in this situation there was a more than twofold increase in the amount of phosphorylated Syk (Figure 2B, graph). Importantly, even at the highest concentration of Stx, the Syk inhibitor piceatannol inhibited Syk phosphorylation (Figure 2B), demonstrating and confirming that the signal observed on anti-PY Western blot is due to a proper Syk kinase activity. Parallel experiments with Syk KD demonstrated that this mutant did not become phosphorylated (Figure 2C), confirming that Stx triggers a Syk activation resulting in its autophosphorylation.
Figure 2.
Autophosphorylation of Syk in response to Stx treatment. HeLa cells transfected with human Syk cDNA and treated with 250 ng/ml Stx for 2.5 or 7.5 min at 37°C (A) or with the indicated concentrations of Stx for 5 min after preincubation with and without piceatannol (P) (50 μM) for 30 min (B). The cells were then lysed, and the lysate was immunoprecipitated overnight with anti-Syk antibody. The immunoprecipitates were analyzed by immunoblotting with mouse anti-PY. The membranes were then stripped and reprobed with mouse anti-Syk antibody. Graph, quantification of the amount of phosphorylated Syk (after normalization for the amount of immunoprecipitated Syk) in these experiments, which are representative experiments of at least three independent experiments. (C) HeLa cells transfected with cDNA encoding for Syk WT or Syk KD were incubated with and without 250 ng/ml Stx for 5 min at 37°C. Cell lysates were analyzed as described in A and B.
Figure 4.
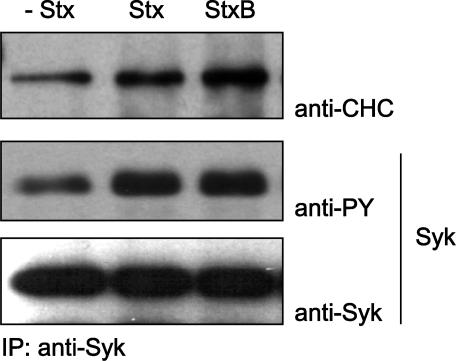
Coimmunoprecipitation of clathrin and Syk. HeLa cells were transfected with Syk WT and treated with 250 ng/ml Stx or Stx B-chain for 5 min at 37°C. The cells were then lysed and immunoprecipitated with anti-Syk antibodies immobilized on protein A/G beads overnight at 4°C. The immunoprecipitates were analyzed by immunoblotting with mouse anti-PY. The membrane was then stripped and reprobed with mouse anti-Syk and then anti-CHC antibodies.
CHC Phosphorylation Is a Result of Syk Activity
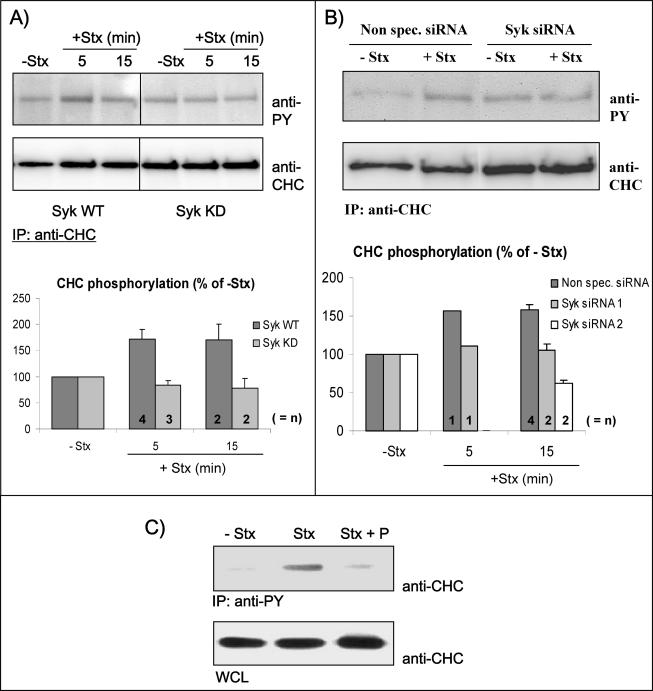
As described above, Syk was activated and clathrin became phosphorylated upon Stx binding. To investigate whether this CHC phosphorylation is a result of Syk activity, we performed an experiment where Syk (WT or KD) was overexpressed, and the cells were stimulated or not by addition of Stx (Figure 3A). Immunoprecipitation of CHC followed by Western blot analysis using anti-PY antibodies revealed a twofold increase in CHC phosphorylation in Syk WT-transfected cells that were stimulated with the toxin (Figure 3A, graph), as also was observed in untransfected cells (Figure 1B, graph). As a negative control, we also immunoprecipitated with nonspecific mouse IgG, which did not give a band at the size of clathrin (our unpublished data). As expected, the kinase-dead mutant of Syk was not able to trigger an increase in the phosphosignal of CHC. Quantification of the amount of clathrin phosphorylation from two to four independent experiments is shown in Figure 3A (graph). These results suggest that the Stx-induced phosphorylation of clathrin is dependent on Syk activity. To further strengthen this suggestion, we have also investigated clathrin phosphorylation in cells depleted of Syk by siRNA. As shown in Figure 3B, depletion of Syk by using Syk siRNA 1 abolished Stx-induced CHC phosphorylation, whereas incubation with Stx increased CHC phosphorylation to 161% in cells transfected with nonspecific siRNA. Real-time PCR revealed that the amount of Syk mRNA was almost completely removed (with 80–90%) in these experiments (see Materials and Methods). To verify that the effect seen by using Syk siRNA 1 was not because of any off-target effects of the oligos, we also checked another siRNA oligo designed against Syk, Syk siRNA 2. Like Syk siRNA 1, also Syk siRNA 2 was able to abolish the Stx induced phosphorylation of clathrin (Figure 3B, graph), suggesting that the effect seen is Syk specific. To further confirm our suggestion that Stx-induced phosphorylation of clathrin is dependent on Syk activity, we also investigated whether the Syk inhibitor piceatannol was able to affect this clathrin phosphorylation. Thus, cells were incubated with Stx, with or without a preincubation with piceatannol, and tyrosine-phosphorylated proteins were then immunoprecipitated by using anti-PY antibodies, and CHC was visualized by Western blot analysis. As shown in Figure 3C, Stx-dependent clathrin phosphorylation was inhibited by piceatannol, strengthening the suggestion that Syk is a likely candidate kinase for CHC phosphorylation. Together, these data support the hypothesis that Syk and CHC are part of the same signaling pathway stimulated upon Stx binding.
Figure 3.
CHC phosphorylation results from Syk activation. (A) HeLa cells transfected with Syk WT or Syk KD were incubated with or without Stx for the different time points. The cells were lysed and clathrin was immunoprecipitated with anti-CHC antibodies overnight at 4°C. Finally, CHC tyrosine phosphorylation was monitored by Western blot analysis. The membrane was stripped and reprobed with anti-CHC to check equal loading of the material. Graph, quantification of the amount of phosphorylated CHC (after normalization for the amount of immunoprecipitated CHC). The error bars show the SEM (n = 3–4) or the deviation (n = 2) between the independent experiments. (B) HeLa cells transfected with nonspecific siRNA or Syk siRNA 1 were incubated with or without Stx (250 ng/ml) for 15 min. The cells were then lysed and immunoprecipitated as described in A. Graph, quantification of the amount of phosphorylated CHC (after normalization for the amount of immunoprecipitated CHC) from several experiments using both Syk siRNA 1 and Syk siRNA 2. The Syk mRNA level was reduced by 80–90 or 70–80% after transfection with siRNA 1 or 2, respectively. The error bars show the SEM (n = 4) or the deviation (n = 2) between the independent experiments. (C) Piceatannol inhibits tyrosine phosphorylation of clathrin. HeLa cells were pretreated with or without piceatannol (P) (50 μM) for 30 min and then incubated with 250 ng/ml Stx for 5 min at 37°C. The cells were then lysed, and proteins with phosphotyrosine were immunoprecipitated by using the slurry of an anti-phosphotyrosine (PY) column overnight at 4°C. The immunoprecipitates were analyzed by immunoblotting with mouse anti-CHC antibody. To show that equal amounts of cells were lysed and used for the immunoprecipitation, WCLs were loaded and analyzed.
Complex Formation between Syk and Clathrin
The Syk kinase has two tandem SH2 domains, a linker domain, and at its C terminus, a catalytic domain. The SH2 domains of Syk are involved in binding to tyrosine-phosphorylated receptors via the so-called immunoreceptor tyrosine-based activation motif (ITAM), and this binding leads to tyrosine kinase activation of Syk (van Oers and Weiss, 1995). Even in HeLa cells that were not incubated with Stx, there is a low level of CHC tyrosine phosphorylation (Figure 1). We decided to investigate whether Syk is able to form a complex with clathrin. By transfecting HeLa cells with Syk wild type, we were able to demonstrate that a stable complex of clathrin and Syk was already present in cells that had not been incubated with Stx but that after addition of Stx or the B-subunit, there seems to be more clathrin present in a complex with Syk (Figure 4). Control experiments on untransfected cells showed that the Syk antibody alone was not able to coimmunoprecipitate CHC (our unpublished data). However, future studies are required to investigate this complex.
Importance of Syk for Endocytosis of Stx
To investigate whether the Stx-induced signaling described above is important for the uptake of the toxin, we investigated the effect of depleting Syk by siRNA on Stx endocytosis. Stx is a highly stable protein, and very little toxin is degraded. Also recycling of Stx plays a minor role during the incubation times used (our unpublished data). As shown in Figure 5A, depletion of Syk by siRNA 1 resulted in a strong inhibition of Stx endocytosis; the effect was greatest at early time points (∼40% reduction after 5 and 10 min compared with ∼20% after 20 min). Quantification of the degree of endocytosis (as percentage of total cell-associated toxin) from two independent experiments (each done in triplicate) after Stx endocytosis for 10 min is also shown (Figure 5A, columns). Also, by using Syk siRNA 2, we were able to observe a decrease in Stx endocytosis, although the effect was slightly less than for Syk siRNA 1 (∼30% reduction after 10 min) (Figure 5A, columns). This might be explained by a slightly lower reduction in Syk mRNA level after transfection with Syk siRNA 2 (70–80%) compared with Syk siRNA 1 (80–90%) (see Materials & Methods), which is not surprising because Syk siRNA 1 is a mixture of four different siRNA oligos against Syk. To strengthen the suggestion that Syk is implicated in Stx endocytosis, we also analyzed the effect of the general tyrosine inhibitor genistein as well as the Syk inhibitor piceatannol, on the toxin uptake. As shown in Figure 5B, both genistein (25 μg/ml) and piceatannol (50 μM) reduced toxin entry during a 20-min period (with ∼40 and 30%, respectively), supporting the idea that Syk is indeed important for Stx endocytosis. Lower concentrations of piceatannol had a smaller inhibitory effect. As expected, the combination of piceatannol and genistein did not give any further reduction than genistein alone (our unpublished data). We also examined Stx endocytosis after shorter times, and incubation with Stx for 10 min after treatment with genistein or piceatannol gave a similar inhibition (40 and 20%, respectively) (our unpublished data). In contrast, SU6656, a highly specific SFK inhibitor, had essentially no effect on Stx uptake (∼10%, even at high concentration) (Figure 5B). Also, PP1 and PP2, two other SFK inhibitors, had no effect on the toxin uptake (our unpublished data). Thus, in contrast to the results reported for BCR (Stoddart et al., 2002), T-cell receptor (TCR) (Crotzer et al., 2004) and EGF receptor (EGFR) (Wilde et al., 1999), Src or a related kinase does not seem to be involved in regulation of Stx endocytosis. The fact that endocytosis of Stx can be reduced by piceatannol to almost the same extent as observed in the presence of genistein suggests that the main tyrosine kinase involved in Stx uptake in HeLa cells is Syk. Furthermore, overexpression of Syk KD inhibited Stx endocytosis by ∼25% compared with transfection with the vector alone (Figure 5C), which is comparable with the reduction observed in cells treated with piceatannol because the estimated transfection efficiency was ∼60–70% in these experiments. Interestingly, overexpression of Syk WT did not increase the uptake of Stx (Figure 5C). This suggests that the amount of endogenous Syk is sufficient to give optimal activity on endocytosis. To further confirm these biochemical data, we have also monitored Stx entry after transfection with Syk (WT or KD) by using confocal microscopy. In this assay, we observed that ∼70% of the cells transfected with Syk KD showed a decrease in Stx entry after 5 min of incubation with the toxin compared with untransfected cells (our unpublished data). In cells overexpressing Syk WT, we could not observe any significant changes.
Figure 5.
Syk is involved in Stx endocytosis. (A) HeLa cells transfected with nonspecific siRNA or Syk siRNA 1 or 2 were incubated with TAG- and biotin-labeled Stx (25 ng/ml) for the indicated time points. To remove the SS-linked biotin on the cell surface-bound toxin, half of the cells were then incubated with 0.1 M MESNa for 20 min on ice. Subsequently, the cells were washed and lysed, and the amount of TAG- and biotin-labeled toxin in the lysates was then measured using streptavidin beads and BioVeris detection system. Stx endocytosis was then plotted as percentage of total cell-associated toxin. Embedded graph, quantification of the degree of endocytosis (as percentage of nonspecific siRNA) after a 10-min incubation period. The Syk mRNA level was reduced by 80–90 or 70–80% after transfection with siRNA 1 or 2, respectively. The error bars show the deviation between two independent experiments, each done in triplicate. (B) HeLa cells preincubated with genistein (25 μg/ml), piceatannol (50 μM), or SU6656 (10 μM) for 30 min at 37°C were treated with TAG- and biotin-labeled Stx for 20 min and further treated as described in A. (C) HeLa cells transfected with the indicated constructs were incubated with TAG- and biotin-labeled Stx for 20 min and further treated as described in A. (D) Ricin and transferrin, both iodinated, were tested for uptake with the indicated inhibitors. Stx data were also plotted to facilitate a comparison. The error bars in A–C show the SEM between at least three independent experiments.
We then tested whether Stx only affects its own endocytosis or whether uptake of other ligands also was changed. To this end, we have measured the uptake of transferrin, which enters via a constitutive clathrin-dependent mechanism, and ricin, which is taken in via both clathrin-dependent and -independent mechanisms. However, neither the uptake of transferrin nor ricin endocytosis was affected by piceatannol or genistein treatment (Figure 5D). We have also performed endocytosis experiments with cells transfected with Syk (WT or KD), and again neither ricin nor transferrin was affected by the kinase (our unpublished data). These data clearly indicate that this Syk-regulated uptake is specific for Stx.
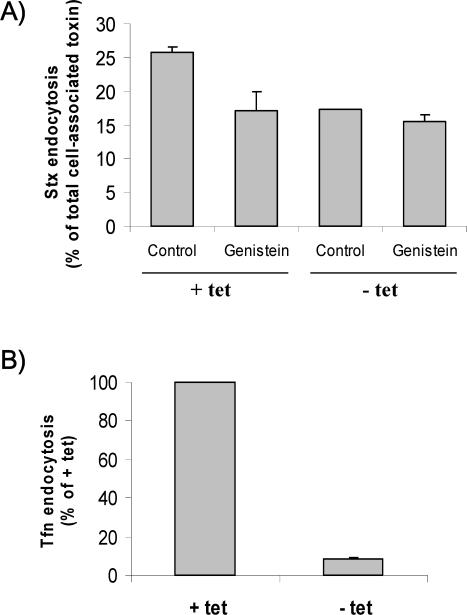
To confirm that the Syk-regulated uptake of Stx occurred via a clathrin-dependent mechanism, we have investigated the effect of genistein on Stx endocytosis in our antisense CHC cells (Iversen et al., 2003). As shown in Figure 6A, genistein inhibits the uptake of Stx only when CHC is expressed (+tet), but in the absence of functional CHC (–tet) genistein had essentially no effect (<10%). To verify that the clathrin-dependent endocytosis was blocked in the absence of tetracycline in our experiments, transferrin endocytosis was analyzed in parallel (Figure 6B). From these data, we can conclude that because a general tyrosine kinase inhibitor is able to selectively affect Stx uptake when functional clathrin is present, CHC-dependent uptake is the only tyrosine kinase-regulated route for Stx. This is also in agreement with the 40% reduction in Stx endocytosis that we observe in Syk siRNA 1 transfected cells—the other 60% most likely representing clathrin-independent endocytosis.
Figure 6.
Effect of genistein on clathrin-dependent and -independent Stx uptake. (A) BHK cells were induced (–tet)) or not (+tet) for the expression of antisense CHC for 2 d and tested in the presence or absence of genistein in a Stx uptake experiment carried out as described in the legend to Figure 5. The degree of endocytosis (as percentage of total cell-associated toxin) was then calculated. The error bars represent deviations between duplicates. The experiment shown is representative of eight independent experiments. (B) To verify that the clathrin-dependent endocytosis was blocked in the absence of tetracycline in our experiments, transferrin endocytosis was analyzed in parallel. Thus, cells were incubating with TAG- and biotin-labeled transferrin (50 ng/ml) for 5 min and further treated as described for Stx.
Involvement of Tyrosine Kinases in the Transport of Stx to the Golgi Apparatus
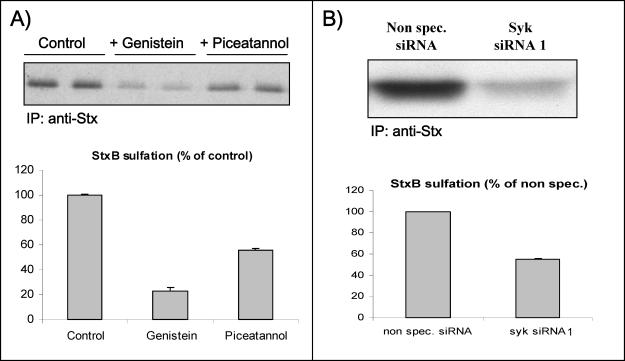
To study the involvement of tyrosine kinases in intracellular transport of Stx, we have used a genetically modified Stx B-chain that can be sulfated (Johannes et al., 1997), a modification that occurs only in the trans-Golgi network (TGN). By using radioactive sulfate, one can quantify transport of StxB to the TGN. As shown in Figure 7A, both the general tyrosine kinase inhibitor genistein and the Syk inhibitor piceatannol reduced the sulfation of StxB. A quantification of bands from three independent experiments (Figure 7A, graph) revealed that Stx B-chain sulfation was reduced to ∼30% of the control level by genistein, whereas piceatannol reduced sulfation with ∼50%. Importantly, we have also examined the effect of StxB sulfation in cells depleted for Syk by siRNA (Figure 7B). Quantification from two independent experiments revealed a 45% decrease in Stx transport to the Golgi apparatus in Syk-depleted cells compared with cells transfected with nonspecific siRNA (Figure 7B, graph), which fits well with the reduction observed with piceatannol. This reduction is also comparable with the one observed in the endocytosis measurements, suggesting that the decreased transport to the TGN is mainly because of reduced toxin endocytosis. Because genistein has a stronger inhibitory effect on StxB sulfation than piceatannol, other tyrosine kinases than Syk might regulate endosome to TGN transport of StxB. The sulfation data support the view that the measurements of toxin endocytosis actually reflect toxin on its way to the Golgi apparatus, and they support the notion that Syk is important for endocytosis.
Figure 7.
Effect of genistein and piceatannol on the sulfation of StxB-Sulf2. (A) HeLa cells were incubated with radioactive sulfate for 3 h at 37°C, and during the last 30 min, 50 μg/ml genistein or 50 μM piceatannol was added (duplicate samples). Then, StxB-Sulf2 (1 μg/ml) was added to the medium, and the incubation was continued for 1 h. The cells were subsequently washed, lysed, and immunoprecipitated with rabbit anti-Stx antibody overnight at 4°C. The adsorbed material was analyzed by 12% SDS-PAGE before autoradiography. The experiment shown is representative of at least three independent experiments. Graph, quantification of the amount of sulfated StxB. The error bars show the deviation between the two duplicates. (B) HeLa cells transfected with nonspecific siRNA or Syk siRNA 1 were incubated with radioactive sulfate and then StxB-Sulf2 as described in A. The Syk mRNA level was reduced by 80–90% after transfection with Syk siRNA 1. Graph, quantification of the amount of sulfated StxB. The error bars show the deviation between two independent experiments.
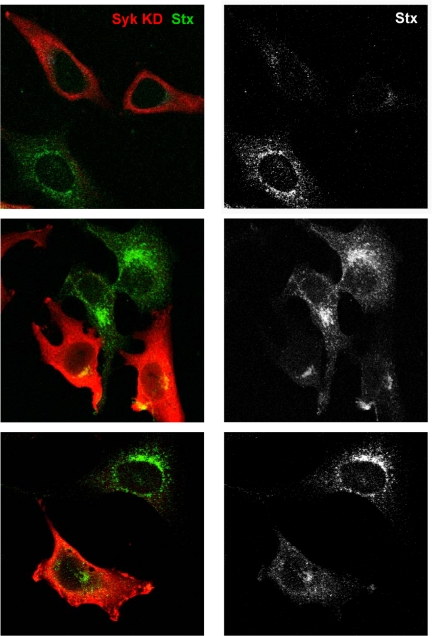
Notably, the conclusion that Syk is important for endocytosis and therefore also for Golgi transport also can be demonstrated by confocal microscopy. Cells overexpressing the mutant kinase Syk KD showed a clear reduction in Stx content in the Golgi area as well as in vesicles compared with untransfected cells (Figure 8). We could not observe any difference in cells transfected with Syk WT compared with untransfected cells (our unpublished data), supporting the proposition that overexpression of Syk does not affect the toxin uptake. A reduced transport to the Golgi apparatus was also observed in cells transfected with Syk siRNA 1 compared with cells transfected with nonspecific siRNA, and quantification from two independent experiments revealed that depletion of Syk reduced the colocalization between Stx and TGN with 62% (our unpublished data).
Figure 8.
Syk KD is able to reduce Stx endocytosis and trafficking to the Golgi apparatus. HeLa cells were transfected 24 h before the experiment. They were then washed and incubated with Stx (250 ng/ml) for 45 min at 37°C. After fixation, the cells were visualized by confocal microscopy. Transfected cells were distinguished by using anti-Syk antibodies (red channel), and Stx was revealed by using anti-Stx antibodies (green channel). The transfected cells take up less Stx and also have less toxin in the Golgi area. In the panels to the right, Stx is shown in gray because this makes it easier to see the effect of the expression of Syk KD on its transport.
DISCUSSION
The main finding presented here is that binding of Stx induces a signaling resulting in Syk activation and CHC tyrosine phosphorylation. The result of this Syk activation seems to be an increase in Stx entry into the cell. In agreement with this idea is the finding that shutting down the synthesis of Syk by Syk siRNA transfection reduces Stx uptake by 40%. Also, when Syk activity is reduced by chemical inhibitors, or a dominant negative form of Syk is overexpressed, endocytosis of Stx is reduced. Because Stx binding increases the amount of Syk found in complex with CHC, the data may suggest that it is Syk itself, and not another kinase, that phosphorylates clathrin. In analogy with other data obtained with EGF, it is tempting to suggest that the toxin-induced phosphorylation of CHC is important for its entry into the cell. The finding that inhibition of tyrosine phosphorylation by genistein only inhibits Stx uptake when clathrin-dependent endocytosis is operating supports this idea. When antisense clathrin was induced and clathrin-dependent endocytosis was blocked, there was no further decrease in clathrin-independent endocytosis by addition of genistein. The mechanisms involved in the clathrin-independent endocytosis of Stx are not known; however, they could involve the recently described CtBP3/BARS-dependent process (Bonazzi et al., 2005). Furthermore, the fact that Syk inhibition gives the same reduction in sulfation of StxB as the inhibition seen in endocytosis suggests that Syk-induced phosphorylation of clathrin might be important for endocytosis of Stx only, and not also for endosome-to-Golgi transport of Stx, although this process also is dependent on clathrin in toxin-sensitive cells (Lauvrak et al., 2004; Saint-Pol et al., 2004). The results also suggest that clathrin-dependent uptake of Stx is important for transport to the Golgi apparatus. There seems to be several pathways between endosomes and the Golgi apparatus, and in the case of Stx the toxin can at high concentrations reach the Golgi apparatus even in the absence of functional clathrin (Lauvrak et al., 2004; Saint-Pol et al., 2004). Toxin transport to the ER could in principle occur without the toxin passing the TGN; however, we do not have any evidence for such a pathway so far when it comes to Stx transport.
Tyrosine phosphorylation of CHC has been reported also under several other circumstances. Tyrosine phosphorylation of CHC occurs upon binding of EGF (Wilde et al., 1999) and nerve growth factor (NGF) (Beattie et al., 2000), during BCR internalization (Stoddart et al., 2002), TCR signaling, and internalization (Crotzer et al., 2004) and during oxidative stress (Ihara et al., 2002), but in these cases phosphorylation is caused by Src kinases, because inhibitors of this group of enzymes were shown to interfere with this process. However, in a recent article by Sorkina et al. (2002), it was reported that although the commonly used Src inhibitor PP2 inhibited EGF uptake, a similar inhibition was not observed with a more specific inhibitor of Src (SU6656), raising the question of whether another kinase was involved in EGF-induced endocytosis. Interestingly, similarly to our data, a basal level of clathrin tyrosine phosphorylation was observed also in Daudi and Ramos cells (Stoddart et al., 2002) and in chicken embryo fibroblasts transformed by Rous Sarcoma virus (Martin-Perez et al., 1989). In vitro assays performed on tyrosine kinases have shown that Syk and Src have different amino acid requirements for substrate specificity (Bewarder et al., 1996; Schmitz et al., 1996; Ruzza et al., 2003). It is therefore likely that Syk induces phosphorylation of different tyrosine residue(s) than Src in clathrin. Indeed, we are currently working on the binding of Syk to CHC, and we have obtained convincing evidence that the phosphorylation site(s) of Syk on CHC is not the same as the one used by Src kinase (our unpublished data). To which extent this has different effects on clathrin and its interacting partner proteins is not known, but it might very well be the case. After H2O2-induced oxidative stress, there is a redistribution of clathrin from the membrane to the cytosol, leading to a reduction of transferrin endocytosis. In contrast, NGF binding increased the amount of clathrin at the cell surface and the uptake of transferrin, and similarly, EGF increased the number of clathrin-coated pits at the cell surface (Wilde et al., 1999). In our studies, we could not measure any difference in transferrin uptake after addition of Stx (our unpublished data), suggesting that the cellular events differ. If clathrin is recruited to the site of Stx binding, then phosphorylated and incorporated into a clathrin-coated pit, these pits do not necessarily contain transferrin receptors to the same extent as those already present, and one might therefore not see an increase in transferrin uptake. In agreement with such an idea are the following results from other laboratories: Cbl mutants affected EGF internalization without inhibiting transferrin uptake (Jiang and Sorkin, 2003) and a dominant negative Eps15 mutant inhibited EGFR endocytosis by retaining the receptors in clathrin-coated pits without affecting endocytosis of transferrin receptors (Confalonieri et al., 2000). Another possibility is that clathrin-coated pits with Stx also contain transferrin, but that these pits just lead to a dilution of transferrin in all clathrin-coated pits present at the membrane. Importantly, as suggested for the BCR receptor (Stoddart et al., 2002), clathrin phosphorylation might be a negative signal for clathrin-coated pit formation. It might actually delay the process, providing the ligand to be internalized with more time to locate to clathrin-coated domains. In our study, we have shown that Stx binding is followed by CHC phosphorylation. However, the increase in phosphorylation caused by the overexpression of Syk does not improve the toxin uptake. Thus, the level of the endogenous enzyme seems to be sufficient to facilitate maximum uptake of Stx. It should be noted that although genistein selectively may inhibit pinching off of caveolae and not clathrin coated pits with prelocalized transferrin receptors (for review, see Marks and Pagano, 2002), inhibition of tyrosine phosphorylation might still inhibit relocalization of a given receptor to clathrin-coated pits. This has been shown to be the case for the EGF receptor (Lamaze et al., 1993) and seems to be the case also for cholera toxin, which can enter from clathrin-coated pits in some cell types (Torgersen et al., 2001).
An important question without any answer so far is: How is Stx able to activate Syk? It is interesting in this connection that also antibodies to Gb3 are reported to cause signaling in lymphocytes, but this signaling is presumably of a different type than what occurs after addition of toxin (Tetaud et al., 2003). Thus, cross-linking of Gb3 may have different consequences for the cell, depending on what is bound. Therefore, it is tempting to suggest that the glycosphingolipid receptor for Stx, Gb3, and the toxin itself or the binding moiety of the toxin (the B-chains) interact with a transmembrane structure able to mediate a signal to the cytosolic side of the membrane. It has been reported that the toxin can interact with other proteins at the cell surface (Shimizu et al., 2003), and the possibility exists that these might be important for signaling. Also, aggregation of Gb3 caused by binding of the pentameric B-moiety, perhaps in lipid rafts, might play a role. In the case of BCR, the binding of antigen to the receptor is associated with transfer of BCR to lipid rafts and then there is an induction of clathrin phosphorylation (Stoddart et al., 2002). Interestingly, there is a larger fraction of clathrin that is phosphorylated in rafts in these cells compared with the rest of the membrane. Similarly, it has been shown that Stx which is internalized in toxin-sensitive cells is associated with lipid rafts, and it is known that receptor internalization and transport to the Golgi apparatus are dependent on the length of the fatty acid (Sandvig et al., 1994; Lingwood, 1999). It is possible that this part of the receptor is important for the signaling described in the present article. Whether the activity of Syk in the case of Stx internalization is dependent on raft structures and the type of lipids that make up Gb3 in different cells would be a question for future studies.
Stx is not unique when it, as a ligand, uses Syk activation as a strategy to facilitate entry. Similarly, antibody receptors (FcR) in macrophages, although they do not have enzymatic activity, are able to activate Syk after cross-linking by antibodies. On antibody binding, an SFK first phosphorylates the receptor on ITAM, and then these phosphorylated motifs are recognized by the SH2 domains of Syk. The binding of Syk to these motifs leads to activation of Syk and signaling. However, in contrast to Stx entry, the FcR and the antibody is then internalized via phagocytosis. The different internalization strategies requiring Syk might be a consequence of the scaffolding proteins involved. Thus, signaling can induce uptake by different endocytic pathways. It should be noted that Syk can be activated by different mechanisms. Interestingly, Syk can be activated through interaction with integrin β cytoplasmic domains by a mechanism that is independent of phosphorylation (Woodside et al., 2001, 2002).
As shown in the present article, Stx entry seems to be regulated by stimulation of a signaling cascade. Endocytosis of the toxin seems to be the first step triggered by Syk tyrosine kinase, whereas other tyrosine kinases might be involved in the regulation of later steps of the toxin journey. Importantly, as shown here, the toxin is an active player in its transport.
Acknowledgments
We are grateful to Elin Rolén, Anne-Grethe Myrann, Jorunn Jacobsen, and Sigrid Skånland for expert technical assistance. Prof. H. Yamamura and Prof. K. Sada (Kobe University, Kobe, Japan) are also acknowledged for the gift of porcine Syk cDNA constructs. This study was supported by the Norwegian Cancer Society, the Norwegian Research Council for Science and the Humanities, the Novo Nordisk Foundation, the Jahre Foundation and Jeanette and Søren Bothners Legacy. S. W. was supported by Federation of European Biochemical Societies and Fond National Suisse de la Recherche Scientifique postdoctoral fellowships.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–08–0766) on December 21, 2005.
Abbreviations used: Stx, Shiga toxin; TGN, trans-Golgi network.
References
- Bache, K. G., Slagsvold, T., and Stenmark, H. (2004). Defective downregulation of receptor tyrosine kinases in cancer. EMBO J. 23, 2707–2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beattie, E. C., Howe, C. L., Wilde, A., Brodsky, F. M., and Mobley, W. C. (2000). NGF signals through TrkA to increase clathrin at the plasma membrane and enhance clathrin-mediated membrane trafficking. J. Neurosci. 20, 7325–7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bewarder, N., Weinrich, V., Budde, P., Hartmann, D., Flaswinkel, H., Reth, M., and Frey, J. (1996). In vivo and in vitro specificity of protein tyrosine kinases for immunoglobulin (Ig) G receptor (FcgammaRII) phosphorylation. Mol. Cell. Biol. 16, 4735–4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolen, J. B., and Brugge, J. S. (1997). Leukocyte protein tyrosine kinases: potential targets for drug discovery. Annu. Rev. Immunol. 15, 371–404. [DOI] [PubMed] [Google Scholar]
- Bonazzi, M., et al.. (2005). CtBP3/BARS drives membrane fission in dynamin-independent transport pathways. Nat. Cell Biol. 7, 570–580. [DOI] [PubMed] [Google Scholar]
- Bonnerot, C., Briken, V., Brachet, V., Lankar, D., Cassard, S., Jabri, B., and Amigorena, S. (1998). Syk protein tyrosine kinase regulates Fc receptor gamma-chain-mediated transport to lysosomes. EMBO J. 17, 4606–4616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherla, R. P., Lee, S. Y., and Tesh, V. L. (2003). Shiga toxins and apoptosis. FEMS Microbiol. Lett. 228, 159–166. [DOI] [PubMed] [Google Scholar]
- Confalonieri, S., Salcini, A. E., Puri, C., Tacchetti, C., and Di Fiore, P. P. (2000). Tyrosine phosphorylation of Eps15 is required for ligand-regulated, but not constitutive, endocytosis. J. Cell Biol. 150, 905–912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crotzer, V. L., Mabardy, A. S., Weiss, A., and Brodsky, F. M. (2004). T cell receptor engagement leads to phosphorylation of clathrin heavy chain during receptor internalization. J. Exp. Med. 199, 981–991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daeron, M. (1997). Fc receptor biology. Annu. Rev. Immunol. 15, 203–234. [DOI] [PubMed] [Google Scholar]
- Di Fiore, P. P., and De Camilli, P. (2001). Endocytosis and signaling. An inseparable partnership. Cell 106, 1–4. [DOI] [PubMed] [Google Scholar]
- Falguieres, T., Mallard, F., Baron, C., Hanau, D., Lingwood, C., Goud, B., Salamero, J., and Johannes, L. (2001). Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 12, 2453–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, G. H., Armstrong, C. S., Sakiri, R., and Tesh, V. L. (2000). Shiga toxin-induced tumor necrosis factor alpha expression: requirement for toxin enzymatic activity and monocyte protein kinase C and protein tyrosine kinases. Infect. Immun. 68, 5183–5189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster, G. H., and Tesh, V. L. (2002). Shiga toxin 1-induced activation of c-Jun NH(2)-terminal kinase and p38 in the human monocytic cell line THP-1, possible involvement in the production of TNF-alpha. J. Leukoc. Biol. 71, 107–114. [PubMed] [Google Scholar]
- Gonzalez-Gaitan, M., and Stenmark, H. (2003). Endocytosis and signaling: a relationship under development. Cell 115, 513–521. [DOI] [PubMed] [Google Scholar]
- Hunter, T. (2000). Signaling–2000 and beyond. Cell 100, 113–127. [DOI] [PubMed] [Google Scholar]
- Ihara, Y., Yasuoka, C., Kageyama, K., Wada, Y., and Kondo, T. (2002). Tyrosine phosphorylation of clathrin heavy chain under oxidative stress. Biochem. Biophys. Res. Commun. 297, 353–360. [DOI] [PubMed] [Google Scholar]
- Iversen, T. G., Skretting, G., van Deurs, B., and Sandvig, K. (2003). Clathrin-coated pits with long, dynamin-wrapped necks upon expression of a clathrin antisense RNA. Proc. Natl. Acad. Sci. USA 100, 5175–5180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, X., and Sorkin, A. (2003). Epidermal growth factor receptor internalization through clathrin-coated pits requires Cbl RING finger and proline-rich domains but not receptor polyubiquitylation. Traffic 4, 529–543. [DOI] [PubMed] [Google Scholar]
- Johannes, L., Tenza, D., Antony, C., and Goud, B. (1997). Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 272, 19554–19561. [DOI] [PubMed] [Google Scholar]
- Katagiri, Y. U., Mori, T., Nakajima, H., Katagiri, C., Taguchi, T., Takeda, T., Kiyokawa, N., and Fujimoto, J. (1999). Activation of Src family kinase yes induced by Shiga toxin binding to globotriaosyl ceramide (Gb3/CD77) in low density, detergent-insoluble microdomains. J. Biol. Chem. 274, 35278–35282. [DOI] [PubMed] [Google Scholar]
- Lamaze, C., Baba, T., Redelmeier, T. E., and Schmid, S. L. (1993). Recruitment of epidermal growth factor and transferrin receptors into coated pits in vitro: differing biochemical requirements. Mol. Biol. Cell 4, 715–727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lauvrak, S. U., Torgersen, M. L., and Sandvig, K. (2004). Efficient endosome-to-Golgi transport of Shiga toxin is dependent on dynamin and clathrin. J. Cell Sci. 117, 2321–2331. [DOI] [PubMed] [Google Scholar]
- Lingwood, C. A. (1999). Glycolipid receptors for verotoxin and Helicobacter pylori: role in pathology. Biochim. Biophys. Acta 1455, 375–386. [DOI] [PubMed] [Google Scholar]
- Majeed, M., Caveggion, E., Lowell, C. A., and Berton, G. (2001). Role of Src kinases and Syk in Fcgamma receptor-mediated phagocytosis and phagosome-lysosome fusion. J. Leukoc. Biol. 70, 801–811. [PubMed] [Google Scholar]
- Marks, D. L., and Pagano, R. E. (2002). Endocytosis and sorting of glycosphingolipids in sphingolipid storage disease. Trends Cell Biol. 12, 605–613. [DOI] [PubMed] [Google Scholar]
- Martin-Perez, J., Bar-Zvi, D., Branton, D., and Erikson, R. L. (1989). Transformation by Rous sarcoma virus induces clathrin heavy chain phosphorylation. J. Cell Biol. 109, 577–584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mori, T., et al. (2000). Globotriaosyl ceramide (CD77/Gb3) in the glycolipid-enriched membrane domain participates in B-cell receptor-mediated apoptosis by regulating lyn kinase activity in human B cells. Exp. Hematol. 28, 1260–1268. [DOI] [PubMed] [Google Scholar]
- Nichols, B. J., Kenworthy, A. K., Polishchuk, R. S., Lodge, R., Roberts, T. H., Hirschberg, K., Phair, R. D., and Lippincott-Schwartz, J. (2001). Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park, J. G., and Schreiber, A. D. (1995). Determinants of the phagocytic signal mediated by the type IIIA Fc gamma receptor, Fc gamma RIIIA: sequence requirements and interaction with protein-tyrosine kinases. Proc. Natl. Acad. Sci. USA 92, 7381–7385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renedo, M. A., Fernandez, N., and Crespo, M. S. (2001). FcgammaRIIA exogenously expressed in HeLa cells activates the mitogen-activated protein kinase cascade by a mechanism dependent on the endogenous expression of the protein tyrosine kinase Syk. Eur. J. Immunol. 31, 1361–1369. [DOI] [PubMed] [Google Scholar]
- Rodal, S. K., Skretting, G., Garred, O., Vilhardt, F., van Deurs, B., and Sandvig, K. (1999). Extraction of cholesterol with methyl-beta-cyclodextrin perturbs formation of clathrin-coated endocytic vesicles. Mol. Biol. Cell 10, 961–974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowley, R. B., Burkhardt, A. L., Chao, H. G., Matsueda, G. R., and Bolen, J. B. (1995). Syk protein-tyrosine kinase is regulated by tyrosine-phosphorylated Ig alpha/immunoglobulin beta immunoreceptor tyrosine activation motif binding and autophosphorylation. J. Biol. Chem. 270, 11590–11594. [DOI] [PubMed] [Google Scholar]
- Ruzza, P., Calderan, A., Donella-Deana, A., Biondi, B., Cesaro, L., Osler, A., Elardo, S., Guiotto, A., Pinna, L. A., and Borin, G. (2003). Conformational constraints of tyrosine in protein tyrosine kinase substrates: information about preferred bioactive side-chain orientation. Biopolymers 71, 478–488. [DOI] [PubMed] [Google Scholar]
- Saint-Pol, A., et al. (2004). Clathrin adaptor epsinR is required for retrograde sorting on early endosomal membranes. Dev. Cell 6, 525–538. [DOI] [PubMed] [Google Scholar]
- Sandvig, K. (2001). Shiga toxins. Toxicon 39, 1629–1635. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., Grimmer, S., Lauvrak, S. U., Torgersen, M. L., Skretting, G., van Deurs, B., and Iversen, T. G. (2002). Pathways followed by ricin and Shiga toxin into cells. Histochem. Cell Biol. 117, 131–141. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., Olsnes, S., Brown, J. E., Petersen, O. W., and van Deurs, B. (1989). Endocytosis from coated pits of Shiga toxin: a glycolipid-binding protein from Shigella dysenteriae 1. J. Cell Biol. 108, 1331–1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K., Ryd, M., Garred, O., Schweda, E., Holm, P. K., and van Deurs, B. (1994). Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J. Cell Biol. 126, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (2002). Membrane traffic exploited by protein toxins. Annu. Rev. Cell Dev. Biol. 18, 1–24. [DOI] [PubMed] [Google Scholar]
- Schmitz, R., Baumann, G., and Gram, H. (1996). Catalytic specificity of phosphotyrosine kinases Blk, Lyn, c-Src and Syk as assessed by phage display. J. Mol. Biol. 260, 664–677. [DOI] [PubMed] [Google Scholar]
- Shimizu, T., Hamabata, T., Yoshiki, A., Hori, T., Ito, S., Takeda, Y., and Hayashi, H. (2003). An association of 27- and 40-kDa molecules with glycolipids that bind A-B bacterial enterotoxins to cultured cells. Biochim. Biophys. Acta 1612, 186–194. [DOI] [PubMed] [Google Scholar]
- Smith, W. E., Kane, A. V., Campbell, S. T., Acheson, D. W., Cochran, B. H., and Thorpe, C. M. (2003). Shiga toxin 1 triggers a ribotoxic stress response leading to p38 and JNK activation and induction of apoptosis in intestinal epithelial cells. Infect. Immun. 71, 1497–1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorkin, A., and Von Zastrow, M. (2002). Signal transduction and endocytosis: close encounters of many kinds. Nat. Rev. Mol. Cell. Biol. 3, 600–614. [DOI] [PubMed] [Google Scholar]
- Sorkina, T., Huang, F., Beguinot, L., and Sorkin, A. (2002). Effect of tyrosine kinase inhibitors on clathrin-coated pit recruitment and internalization of epidermal growth factor receptor. J. Biol. Chem. 277, 27433–27441. [DOI] [PubMed] [Google Scholar]
- Stoddart, A., Dykstra, M. L., Brown, B. K., Song, W., Pierce, S. K., and Brodsky, F. M. (2002). Lipid rafts unite signaling cascades with clathrin to regulate BCR internalization. Immunity 17, 451–462. [DOI] [PubMed] [Google Scholar]
- Strzelecka-Kiliszek, A., Kwiatkowska, K., and Sobota, A. (2002). Lyn and Syk kinases are sequentially engaged in phagocytosis mediated by Fc gamma R. J. Immunol. 169, 6787–6794. [DOI] [PubMed] [Google Scholar]
- Su, G. F., Brahmbhatt, H. N., Wehland, J., Rohde, M., and Timmis, K. N. (1992). Construction of stable LamB-Shiga toxin B subunit hybrids: analysis of expression in Salmonella typhimurium aroA strains and stimulation of B subunit-specific mucosal and serum antibody responses. Infect. Immun. 60, 3345–3359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teis, D., and Huber, L. A. (2003). The odd couple: signal transduction and endocytosis. Cell Mol. Life Sci. 60, 2020–2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tetaud, C., et al. (2003). Two distinct Gb3/CD77 signaling pathways leading to apoptosis are triggered by anti-Gb3/CD77 mAb and verotoxin-1. J. Biol. Chem. 278, 45200–45208. [DOI] [PubMed] [Google Scholar]
- Torgersen, M. L., Skretting, G., van Deurs, B., and Sandvig, K. (2001). Internalization of cholera toxin by different endocytic mechanisms. J. Cell Sci. 114, 3737–3747. [DOI] [PubMed] [Google Scholar]
- van Oers, N. S., and Weiss, A. (1995). The Syk/ZAP-70 protein tyrosine kinase connection to antigen receptor signalling processes. Semin. Immunol. 7, 227–236. [DOI] [PubMed] [Google Scholar]
- Walchli, S., Espanel, X., Harrenga, A., Rossi, M., Cesareni, G., and van Huijsduijnen, R. H. (2004). Probing protein-tyrosine phosphatase substrate specificity using a phosphotyrosine-containing phage library. J. Biol. Chem. 279, 311–318. [DOI] [PubMed] [Google Scholar]
- Wilde, A., Beattie, E. C., Lem, L., Riethof, D. A., Liu, S. H., Mobley, W. C., Soriano, P., and Brodsky, F. M. (1999). EGF receptor signaling stimulates SRC kinase phosphorylation of clathrin, influencing clathrin redistribution and EGF uptake. Cell 96, 677–687. [DOI] [PubMed] [Google Scholar]
- Woodside, D. G., Obergfell, A., Leng, L., Wilsbacher, J. L., Miranti, C. K., Brugge, J. S., Shattil, S. J., and Ginsberg, M. H. (2001). Activation of Syk protein tyrosine kinase through interaction with integrin beta cytoplasmic domains. Curr. Biol. 11, 1799–1804. [DOI] [PubMed] [Google Scholar]
- Woodside, D. G., Obergfell, A., Talapatra, A., Calderwood, D. A., Shattil, S. J., and Ginsberg, M. H. (2002). The N-terminal SH2 domains of Syk and ZAP-70 mediate phosphotyrosine-independent binding to integrin beta cytoplasmic domains. J. Biol. Chem. 277, 39401–39408. [DOI] [PubMed] [Google Scholar]
- Yanagi, S., Inatome, R., Takano, T., and Yamamura, H. (2001). Syk expression and novel function in a wide variety of tissues. Biochem. Biophys. Res. Commun. 288, 495–498. [DOI] [PubMed] [Google Scholar]