Abstract
The Saccharomyces cerevisiae SUV3 gene encodes the helicase component of the mitochondrial degradosome (mtEXO), the principal 3′-to-5′ exoribonuclease of yeast mitochondria responsible for RNA turnover and surveillance. Inactivation of SUV3 (suv3Δ) causes multiple defects related to overaccumulation of aberrant transcripts and precursors, leading to a disruption of mitochondrial gene expression and loss of respiratory function. We isolated spontaneous suppressors that partially restore mitochondrial function in suv3Δ strains devoid of mitochondrial introns and found that they correspond to partial loss-of-function mutations in genes encoding the two subunits of the mitochondrial RNA polymerase (Rpo41p and Mtf1p) that severely reduce the transcription rate in mitochondria. These results show that reducing the transcription rate rescues defects in RNA turnover and demonstrates directly the vital importance of maintaining the balance between RNA synthesis and degradation.
INTRODUCTION
The degradation of RNA is an essential element in the expression of genetic information. It is required to control RNA abundance and thus gene expression and to eliminate aberrant or defective molecules that inevitably form during RNA synthesis and maturation (RNA surveillance) (Vasudevan and Peltz, 2003). The posttranscriptional mechanisms affecting mitochondrial gene expression, including RNA turnover, are of particular importance because transcriptional control is relatively simple and rudimentary. The single RNA polymerase (RNAP) of Saccharomyces cerevisiae mitochondria is composed of only two nuclear-encoded protein subunits—the core enzyme encoded by the RPO41 gene and a transcription initiation factor encoded by the MTF1 gene (Masters et al., 1987; Jang and Jaehning, 1991). The RNAP holoenzyme recognizes a simple nonanucleotide promoter sequence (Osinga et al., 1982; Mangus et al., 1994) and initiates synthesis of at least 13 primary multicistronic transcripts that undergo extensive processing to form mature RNAs (Christianson and Rabinowitz, 1983; Tzagoloff and Myers, 1986; Foury et al., 1998; Gagliardi et al., 2004; Schafer, 2005). Such organization leaves little room for regulation at the transcription initiation level and makes posttranscriptional processes, including RNA degradation, key control points for mitochondrial gene expression.
The enzymes controlling RNA turnover in cells and organelles show great evolutionary divergence and are only partially conserved between mitochondria of different organisms (Gagliardi et al., 2004). The enzymatic activity responsible for turnover is, however, on the basic level, similar in all the systems discovered so far—it is that of a 3′-to-5′ processive exoribonuclease, either hydrolytic or phosphorolytic. In most cases, the exoribonuclease activity is contained in a larger multiprotein complex that, in addition to exoribonucleases, also contains RNA helicases, and in certain cases, endonucleases. A model example of such a complex is the eubacterial degradosome (Carpousis, 2002).
The first mitochondrial RNA degradation enzymatic complex was described in yeast S. cerevisiae and named mtEXO, or the mitochondrial degradosome (Margossian and Butow, 1996; Dziembowski et al., 2003). The mitochondrial degradosome is the main exoribonuclease in yeast mitochondria, which, unlike bacteria and animal or plant mitochondria, lack the phosphorolytic polynucleotide phosphorylase activity. Like the bacterial degradosome it contains an RNase and an RNA helicase; the subunit composition is, however, markedly different (Gagliardi et al., 2004). The yeast mitochondrial degradosome is composed of only two protein subunits—an RNR (RNase II-like) superfamily exoribonuclease encoded by the DSS1/MSU1 (YMR287C) gene (Dmochowska et al., 1995) and an NTP-dependent RNA helicase related to the DExH superfamily, encoded by the SUV3 (YPL029W) gene (Stepien et al., 1992). The two proteins are tightly associated and the activity of both is essential for the functioning of the complex. The mitochondrial degradosome is capable of unwinding dsRNA regions and subsequently degrading single-stranded RNA in a 3′-to-5′ direction (Dziembowski et al., 2003). The mtEXO complex interacts with the mitochondrial ribosome (Dziembowski et al., 2003). Genetic interactions link the mitochondrial degradosome with the protein encoded by the PET127 gene, which is involved in 5′ processing and turnover of mitochondrial RNAs (Wiesenberger and Fox, 1997; Wegierski et al., 1998), and with the 5′ untranslated region of the CYTB mRNA (Chen et al., 1999).
Inactivation of either the SUV3 or DSS1 gene gives a similar phenotype, corresponding to complete depletion of mitochondrial degradosome function. SUV3-deficient strains are all strictly respiratory deficient and rapidly lose wild-type mitochondrial DNA (mtDNA) converting to rho–/rho0 forms. Introduction of intronless mtDNA (Seraphin et al., 1987) into the suv3Δ background (strain suv3Δ Δi) does not rescue the respiratory-deficient phenotype; however, it markedly improves the stability of such mtDNA to ∼60% rho+ in an overnight YP-glucose culture (Dmochowska et al., 1995; Golik et al., 1995; Stepien et al., 1995).
Among the plethora of molecular defects found in suv3Δ strains are overaccumulation of excised group I intronic sequences coupled with the destabilization of mature transcripts (Stepien et al., 1992, 1995; Golik et al., 1995; Margossian et al., 1996), accumulation of RNAs with abnormal 5′ and 3′ termini and of high-molecular-weight RNA precursors, variations in steady-state levels of mature transcripts, and disruption of mitochondrial translation (Dziembowski et al., 2003). Together, these phenotypes suggest that the mtEXO complex is the primary activity responsible for RNA degradation and surveillance in yeast mitochondria.
A spontaneous suppressor allowing the suv3ΔΔi strain to grow on respiratory carbon sources was first described in the original suv3Δ deletion mutant (Dmochowska et al., 1995; Golik et al., 1995; Stepien et al., 1995). The suppressor mutation, termed suB9, partially restored respiratory growth to the suv3Δ strain, as long as the mitochondrial genome did not contain the LSU-rRNA gene intron omega (Stepien et al., 1995) or more than three introns in CYTB and COX1 genes (Golik et al., 1995). The suB9 suppressor was found to be nuclear and monogenic, no further characterization was made at that time.
In this work, we show that mutations partially rescuing the phenotype associated with the loss of mitochondrial degradosome caused by disruption of the SUV3 gene, including the suB9 mutation, are point mutations in genes encoding subunits of the mitochondrial RNA polymerase that severely reduce the transcription rate. This is the first direct demonstration that maintaining the balance between RNA synthesis and degradation is crucial for the correct functioning of the mitochondrial genetic system.
MATERIALS AND METHODS
Strains, Media, and Classical Yeast Techniques
The S. cerevisiae strains used in this study are listed in Table 1. Standard yeast media and basic genetic methods were as described previously (Dujardin et al., 1980; Burke et al., 2000). The normal growth temperature was 30°C, and temperature sensitivity was tested at 36°C. The Singer MSM series 200 System micromanipulator (Singer Instruments, Watchet, Somerset, United Kingdom) was used for tetrad dissection and for the isolation of zygotes in isogenic crosses. Yeast were transformed using either the rapid or high-efficiency LiAc/SS-DNA/PEG protocol (Gietz and Woods, 2002).
Table 1.
S. cerevisiae strains used in this study
| Genotype
|
|||
|---|---|---|---|
| Name | Nuclear | Mitochondrial | Origin |
| BWG1 | MATa, his1, ade1, leu2, ura3 | rho+ | Stepien et al. (1992) |
| suv3Δ Δi | MATa, his1, ade1, leu2, ura3, suv3::URA3 | rho+, intronless | Stepien et al. (1995) |
| W303/A/520 | MATa, ade2, trp1, ura3, leu2, his3 | rho+, intronless | J. Lazowska |
| WSU0 Δi | MATa, ade2, trp1, ura3, leu2, his3, suv3::URA3 | rho+, intronless | This work |
| D273-10B/51 | MAT α, ade5 | rho0 | Groudinsky et al. (1981) |
| Y12799 | MAT α, his3, leu2, lys2, ura3, SUV3::kanMX4 | rho-/rho0 | EUROSCARF |
| SUD1 | MATa, ade2, trp1, ura3, leu2, his3, suv3::KanMX4 | rho+, intronless | This work |
| SDB9 | MATa, his1, ade1, leu2, ura3, suv3::URA3, su1-1 | rho+, intronless | Stepien et al. (1995) |
| SUX1 | MATa, ade2, trp1, ura3, leu2, his3, suv3::URA3, su1-2 | rho+, intronless | This work |
| SUX3 | MATa, ade2, trp1, ura3, leu2, his3, suv3::URA3, su2 | rho+, intronless | This work |
| αKxNO41 Δi | MAT α, Kar1-1, trp5, his4, ade6 | rho+, intronless | Stepien et al. (1995) |
| SP55-11 | MAT α, lys2, ura3, suv3::URA3 | rho+, intronless | P. P. Stepien |
| W303-1B/520 | MAT α, ade2, trp1, ura3, leu2, his3 | rho+, intronless | This work |
| DSW3-12/A | MATa, ade2, trp1, ura3, leu2, his3, mtf1su2 | rho+, intronless | This work |
| DSW1-16/C | MATa, ade2, trp1, ura3, leu2, his3, rpo41su1 | rho+, intronless | This work |
Construction and Initial Characterization of suv3Δ Strains
The first suv3Δ mutants were constructed in the background of the BWG1 strain of S. cerevisiae (see Table 1 for all strain genotypes) as complete or partial deletions with the URA3 cassette (Stepien et al., 1992). Because we planned to use such a strain as a starting point in the search for possible suppressors, we decided to recreate this system in the background of another, much better characterized strain W303, which has been extensively used in the study of nucleomitochondrial interactions. From a strain carrying the shorter (d2) deletion, we amplified the URA3 cassette with ∼200 base pairs and ∼100 base pairs SUV3 flanks at the 5′ and 3′ end, respectively, using primers 9090 and 9091. The PCR product was used to transform the W303/A/520 strain, which has the W303 nuclear background combined with the intronless mtDNA. The resulting strain, termed WSU0 Δi, behaved identically to the original suv3Δ strains, becoming respiratory deficient with a moderate decrease in the stability of the mitochondrial genome. To verify whether the partial nature of the deletion could have any influence on the phenotype, we also prepared an isogenic complete deletion strain by replacing the entire SUV3 open reading frame (ORF) with the KanMX4 selection module. We used DNA from the strain Y12799 originating from the Saccharomyces Genome Deletion Project (Winzeler et al., 1999; Giaever et al., 2002), which harbors the SUV3 deletion, as a template for PCR using the SUV3_A and SUV3_D primers. This gave a product containing the KanMX4 module with yeast genomic flanks upstream from the ATG and downstream from the termination codon of SUV3. Transformation of the W303/A/520 strain with this product gave a strain, termed, SUD1, carrying a deletion of the entire SUV3 ORF in the W303 background with the intronless mtDNA. In all our phenotypic analyses and in subsequent suppressor experiments, this strain behaved exactly like the partial deletion generated previously. We can therefore conclude that all the suv3Δ strains we constructed and used were functionally equivalent and corresponded to a complete loss of SUV3 gene function.
PCR Sequencing and Related Techniques
Yeast total DNA for PCR and plasmid isolation was prepared using the rapid phenol/glass beads protocol (Hoffman and Winston, 1987). Primers 9090 (5′-AACTGCGGTTACATGGCCTA) and 9091 (5′-CTCGAAGATGAGAGGTGACC) were used to amplify the suv3::URA3 d2 disruption cassette (Stepien et al., 1992). The suv3::KanMX deletion construct was amplified using primers SUV3_A (5′-TCAGAACACAATGTCCTTATTGAAA) and SUV3_D (5′-TATATTTTACTGCCCTTTGCTCAAC).
Primers MTF_A (5′-GATTATTGCGACTAATTTGAATGGT) and MTF_D (5′-CCTTTTCTTAAAGTTTTAGTTCCGC) were used to amplify the MTF1 gene, and primers MTF_C (5′-CAGTAGTAAGGGAGGCATTTACAGA), MTF_B (5′-AGTTCTTGTTTCCAATACAGGACAG), MTF350F (5′-TATTTGTTCCTGAAGTTCAAT), and MTF690R (5′-GTAGGCCATATTTCCGCAGCA) were used to sequence the MTF1 ORF.
Primers 976L (5′-CCACCAGCTTGTGAATAGGTT), 1764R (5′-CGAGCTTGTCGTTGAATGGA), 1724L (5′-ACTTCCATTCAACGACAAGC), 2427R (5′-CTCTCTTGCGGCTTCCGTTG), 2321L (5′-CACTGCCAACATTAGAGGAA), 3039R (5′-AGCTGGAGCTTCACCGTGAA), 2971L (5′-GTCGCTAAGGTATCTGTGCA), 3733R (5′-ACCACTTGAGACCAGAAGGT), 3714L (5′-ACCTTCTGGTCTCAAGTGGT), 4488R (5′-TAGTCCTAGTGGTGTTCGTCC), 4453L (5′-ATGTCATCCGTCATATGGAC), and 5210R (5′-TTGTAGTTCACGGCTCACGA) were used in pairs to amplify and sequence the RPO41 gene.
Vectors and Genomic Libraries
The wild-type S. cerevisiae genomic library in the pRS200 (ARS-CEN,TRP1) plasmid vector (Sikorski and Hieter, 1989) constructed by P. Hieter was kindly donated by Dr. M. Johnston (Washington University, St. Louis, MO). YCplac111 (ARS-CEN, LEU2) and YCplac33 (ARS-CEN, URA3) (Gietz and Sugino, 1988) were used as vectors. Plasmids pJJ1148 and pJJ1149 (Cliften et al., 2000) containing the RPO41 gene cloned in the YCplac33 (ARS-CEN, URA3) and YCplac111 vectors, respectively, were kindly donated by Dr. Judith Jaehning (University of Colorado, Denver, CO).
RNA Preparation and Northern Hybridization
RNA was prepared from yeast mitochondria purified from log phase liquid cultures by differential centrifugation as described previously (Dziembowski et al., 2003). RNA was run on a 0.8% agarose-formaldehyde gel and blotted onto nylon membrane as described previously (Tomecki et al., 2004). Amount of mitochondrial protein (quantified by the Bradford assay) in the starting preparation was used to normalize the amount of RNA in each lane. Oligonucleotide probes 14S (5′-TATAAGCCCACCGCAGGTTCCCCTACGGTAACTGTA) and CYTB (5′-TATCTATGTATTAATTTAATTATATATTATTTATTAACTCTACCGAT) (Dziembowski et al., 2003) were used to detect the 14S rRNA and CYTB mRNA, respectively.
In Organello Transcription Assay
Isolation of yeast mitochondria and run-on transcription in organello was performed essentially as described previously (Krause and Dieckmann, 2004). Transcription reactions were performed in a buffer containing 50 mM HEPES-KOH, pH 8.0, 10 mM MgCl2, 25 mM KOAc, 10 mM dithiothreitol, 125 μM CTP, ATP, and GTP each, 50 μCi of [α-32P]UTP, 40 U of RiboLock RNase inhibitor (MBI Fermentas, Hanover, MD) and 5 μg of mitochondrial protein (as quantified by the Bradford assay) in a total volume of 50 μl. Two 2-μl aliquots were taken at each time point and spotted onto DEAE-cellulose filter discs (DE81; Whatman, Maidstone, United Kingdom). One of each pair of filters was washed four times in excess 0.4 M Na2HPO4 to remove unincorporated label, and the radioactivity was determined by liquid scintillation. The ratio of radioactive signal on the washed versus unwashed filter determined the incorporation for a given time point.
RESULTS
Spontaneous, Nuclear, Monogenic Suppressor Mutations Partially Restore Respiratory Competence in suv3Δ Strains Carrying the Intronless Mitochondrial Genome
In this work, we have undertaken a systematic study of spontaneous suppressors of the suv3Δ respiratory-deficient phenotype in the context of the intronless mitochondrial genome. Saturated liquid YPD cultures of the WSU0 Δi strain, described above, were plated on YPG plates at ∼2.5 × 107 cells/plate and grown at 30°C for 7 d. Mitochondrial genome integrity was confirmed for each initial culture by crossing to the rho0 tester strain D273-10B/51. Colonies of respiratory-positive pseudorevertants were observed on each plate, with a median frequency of ∼25 colonies/plate, which corresponds to an approximate mutation rate of 1 × 10–6 (Figure 1A). Both large and small pseudorevertant colonies were observed on each plate.
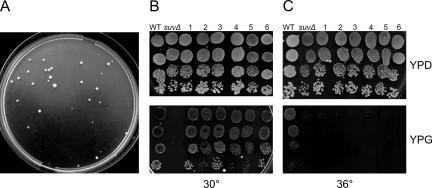
Figure 1.
Analysis of the suppressors of the suv3Δ phenotype. (A) Appearance of the [Gly+] pseudorevertant colonies in the intronless suv3Δ strain after 7 d of incubation on YPG at 30°C. (B) Selected pseudorevertant strains (numbered 1–6) grow at 30°C both on YPD (glucose) and on YPG (glycerol), albeit slower than the wild-type (WT) isogenic control W303/A/520 strain. The suv3Δ parental strain is strictly glycerol negative. (C) The pseudorevertant strains (1–6) fail to grow on YPG at 36°C, in contrast to the WT control. Four 10-fold serial dilutions, beginning with the 10–1 dilution of the saturated liquid YPD preculture are arranged vertically on each panel. Plates were grown for 3 (YPD) or 4 d (YPG).
Subsequent testing showed that all the pseudorevertant strains grew on respiratory media (glycerol) at normal temperature (30°C), albeit visibly slower than the wild-type W303/A/520 strain (Figure 1B). Interestingly, the respiratory-positive phenotype was totally lost from the pseudorevertant strains grown at a higher temperature (36°), which means that the suppression is a temperature-sensitive phenomenon (Figure 1C). Growth on YPD media was not affected at either temperature. Four of the pseudorevertant strains, chosen randomly from among both large and small colony classes, and named SUX1, SUX2, SUX3, and SUX4, were selected for further detailed analysis.
To verify whether the suppressor mutation was carried in the nuclear genome or in the mitochondrial DNA, the four selected pseudorevertant clones were converted to rho0 forms using ethidium bromide and fresh intronless mtDNA was introduced by cytoduction using the strain αKxNO41 (Seraphin et al., 1987) as a donor. In all cases, the respiratory-positive phenotype was maintained in the cytoductants, suggesting that the suppressor mutations occurred in the nuclear genome.
Each pseudorevertant strain was then crossed to the SP55-11 strain (MATα, suv3Δ). The resulting diploids were homozygous for the suv3Δ deletion and heterozygous for the suppressor mutation. Each of the diploids failed to grow on respiratory media, indicating that the suppressor mutations were recessive.
To determine the number of different complementation groups among the suppressor mutations, the MATα suv3Δ su spores obtained by outcrossing the pseudorevertant strains to the wild-type isogenic strain (see next section) were crossed to each of the original pseudorevertant strains. Because the suppressor mutations are recessive, the resulting diploids would be respiratory competent only if both suppressor mutations occurred at the same locus. The results of this analysis indicated that there were at least two different complementation groups of the suppressor mutations, one (larger colonies, strains SUX1 and SUX2) corresponding to the original subB9 mutation, and the other (smaller colonies, SUX3 and SUX4) corresponding to another locus. They were given temporary names of su1 and su2, respectively. The strains carrying the su1 (SUX1) and su2 (SUX3) mutations were selected for further analysis. To summarize, the genetic analysis of the spontaneous suppressors of the suv3Δ disruption showed that the suppressors were nuclear, recessive, and monogenic and they occurred at two distinct loci in the genome. The recessive character of the suppressor mutations practically eliminated the conventional strategy of cloning the suppressor alleles based on their capability of restoring respiration to a suv3Δ strain.
The Suppressors of the suv3Δ Disruption Are Temperature-sensitive Partial Loss-of-Function Mutations in the Mitochondrial RNA Polymerase Genes RPO41 and MTF1
We crossed the pseudorevertant strains to the isogenic MATα wild-type W303-1B/520 strain. Diploids were isolated by micromanipulation and, following sporulation, tetrads were analyzed. This was essentially a two-point cross of suv3::URA3, su× SUV3+, SU+. The suv3Δ spores are readily identified by their [Ura+] phenotype, and the fact that the presence of the suppressor allele gives the suv3Δ spores ability to grow on glycerol at normal temperature facilitated the unambiguous assignment of each tetrad to one of the three classes. The ratio of PD:NPD:TT tetrads in each cross was close to 1:1:4, meaning that the suppressors were monogenic and not linked to the SUV3 locus or to the centromere.
In the NPD tetrads from the crosses described above, two spores carry the suv3Δ allele with the wild-type allele of the suppressor (SU1+ or SU2+) and have the [Ura+ Gly–] phenotype, whereas the other two are SUV3+ and carry the suppressor mutation. Two such NPD tetrads, DSW1-16 and DSW3-12, with the su1 and su2 mutations, respectively, were selected for further phenotypic analysis along with two TT tetrads DSW1-2 and DSW3-1. The spores were tested for uracil auxotrophy (as a marker of the suv3::URA3 disruption) and for growth on glucose (YPD) and glycerol (YPG) media both at normal and elevated (36°C) temperature. Presence of the suv3::URA3 disruption was additionally verified by PCR using primers 9090 and 9091. The results are shown in Figure 2. The suv3Δ, SU+ spores are [Ura+] and [Gly–] at both temperatures, as expected. The [Ura–] SUV3+, su spores are [Gly+] at the normal temperature, although their growth seems to be slower in comparison with a wild-type control. However, at the elevated temperature these spores become strictly [Gly–]. This means that both suppressor mutations give a temperature-sensitive respiratory-deficient phenotype in the context of the wild-type SUV3+ allele. In fact, the respiratory phenotype of these mutant strains is similar to that of the original pseudorevertant strain, regardless of the presence of the functional SUV3 allele. The loss of respiratory function at the elevated temperature is irreversible, mutant cultures grown overnight in liquid YPD at 36°C do not recover a [Gly+] phenotype upon subsequent transfer to 30°C, presumably because they lose mitochondrial DNA.
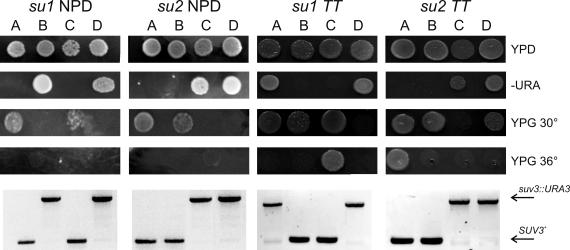
Figure 2.
Analysis of typical TT and NPD tetrads resulting from the cross of pseudorevertant strains SUX1 (su1 mutation) and SUX3 (su2 mutation) with an isogenic wild-type strain W303-1B/520. Tetrads DSW1-16 and DSW1-2 were selected as su1 NPD and su1 TT, respectively. Tetrads DSW3-12 and DSW3-1 were selected as su2 NPD and su2 TT, respectively. Spores were precultured overnight in liquid YPD, 10–1 dilutions of each preculture were spotted on YPD (complete glucose), SC-URA (synthetic complete–uracil), and YPG (complete glycerol) and grown for 3 (YPD, SC-URA) or 4 d (YPG) at 30 and 36°C. Presence of the suv3::URA3 disruption was also verified by PCR (primers 9090 and 9091), with the longer and shorter products corresponding to disruption and wild-type SUV3 allele, respectively. In the NPD tetrads, spores DSW1-16/A and DSW1-16/C contain the su1 mutation and the wild-type SUV3, whereas spores DSW3-12A and DSW13-12/B contain the su2 mutation and the wild-type SUV3.
This temperature-sensitive respiratory-deficient phenotype was used to design a screen for cloning of the suppressor genes. Strain DSW3–12/A was transformed with a wild-type S. cerevisiae genomic library in the pRS200 (ARS-CEN,TRP1) plasmid vector (see Materials and Methods). The [Trp+] transformants were screened for the ability to grow on YPG (glycerol) at 36°C. Ten such colonies were picked for further characterization and were all found to contain the same plasmid clone with an 8.4-kb insert corresponding to a fragment of chromosome XIII and covering two ORFs—RRP5 (YMR229C) and MTF1 (YMR228W). A 1.5-kb SacI-EcoRI fragment containing the MTF1 ORF and a 6.9-kb BamHI-EcoRI fragment containing the RRP5 ORF were subcloned into the YCplac111 (ARS-CEN, LEU2) plasmid vector to yield pMTF1 and pRRP5, respectively. Transformation of the DSW3-12/A strain with the pRRP5 plasmid did not restore respiratory growth at 36°C, whereas transformation with the pMTF1 plasmid restored wild-type respiratory growth at either temperature (Figure 3A). This indicates that the su2 mutation in DSW3-12/A occurred in the MTF1 gene, which encodes the transcription factor of the mitochondrial RNA polymerase (Schinkel et al., 1987; Jang and Jaehning, 1991). Temperature-sensitive respiratory-deficient mutants with defects in MTF1 had been previously described, along with similar mutations in the core mitochondrial RNA polymerase gene RPO41 (Shadel and Clayton, 1995; Cliften et al., 1997, 2000; Karlok et al., 2002; Matsunaga and Jaehning, 2004). We decided therefore to verify directly whether the other suppressor mutation, su1, corresponded to the RPO41 gene. The DSW1-16/C strain, carrying the su1 mutation, was transformed with plasmids pJJ1148 or pJJ1149 (Cliften et al., 2000) containing the wild-type RPO41 gene cloned in the YCplac33 (ARS-CEN, URA3) and YCplac111 vectors, respectively. All the obtained transformants were [Gly+] at both 30 and 36°C (Figure 3B), indicating that the su1 mutation occurred in the RPO41 gene. Passage of the DSW1-16/C/pJJ1148 transformant on fluoroorotic acid media led to the loss of respiratory growth at 36°C concomitant with the loss of uracil prototrophy, which further strengthened the evidence for the association of the su1 suppressor with a mutation in the RPO41 gene.
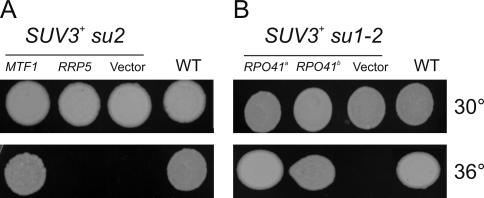
Figure 3.
Rescue of the wild-type (WT) respiratory growth on YPG at 36°C in SUV3+ strains carrying su1 and su2 mutations by low copy number plasmids carrying wild-type alleles of the respective genes. (A) Respiratory growth at 36°C in the SUV3+ su2 strain DSW3–12A is restored by pMTF1 carrying the MTF1 gene, but not by pRRP5 or the empty vector (YCpLac111), to a level observed in the isogenic wild-type control W303/A/520 (WT). (B) Both plasmids carrying the wild-type RPO41 gene on aYCpLac33 (pJJ1148) and bYCpLac111 (pJJ1149), but not the empty vector (YCplac111) restore WT growth at 36°C in the SUV3+ su1 strain DSW1-16/C.
To further verify the assignment of su1 and su2 suppressors to mutations in RPO41 and MTF1, we transformed the original pseudorevertant strains (suv3Δ, su) with plasmids pJJ1149 and pMTF1, carrying wild-type alleles of the respective genes. Because the suppressor alleles were shown to be recessive, introduction of the wild-type allele of the cognate gene should reverse the suppression and revert the transformant to respiratory deficiency at normal temperature. The transformants were tested on synthetic complete media (SC-leucine) with glycerol. The results (Figure 4) indicate that transforming the SUX1 strain with pJJ1149 (RPO41) and transforming the SUX3 strain with pMTF1 results in reversion of the suppressor phenotype, indicating that the plasmid-borne genes are indeed wild-type alleles of the suppressors. The original suppressor strain SDB9 (Stepien et al., 1995) transformed with pJJ149 also reverts to the [Gly–] phenotype, which demonstrates that the suB9 mutation therein occurred in the RPO41 gene.
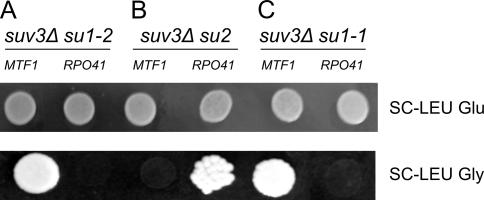
Figure 4.
Plasmid-borne wild-type alleles of MTF1 or RPO41 genes reverse the recessive suppression in suv3Δ pseudorevertant strains with su1 and su2 suppressor mutations. The suppressor phenotype was tested on synthetic complete (SC –leucine) media with a respiratory carbon source (glycerol). (A) Wild-type RPO41 (pJJ1149), but not MTF1 (pMTF1), reverses the suppressor mutation phenotype in the SUX1 strain carrying the su1-2 mutation. (B) Wild-type MTF1 (pMTF1), but not RPO41 (pJJ1149), reverses the suppressor mutation phenotype in the SUX3 strain carrying the su2 mutation. (C) Wild-type RPO41 (pJJ1149), but not MTF1 (pMTF1), reverses the suppressor mutation phenotype in the SDB9 strain carrying the su1-1 (suB9) mutation. Dilutions (10–1) of a saturated preculture were incubated on plates for 5 d.
Because transformation of the pseudorevertant strain with plasmids carrying wild-type alleles of RPO41 or MTF1 is a quick way of determining the nature of suppression, we applied this strategy to a batch of eight additional suppressors isolated in the initial screen and assigned them to mutations in either RPO41 (5 colonies) or MTF1 (3 colonies). Similarly, spontaneous suppressors obtained in the SUD1 strain (carrying the KanMX4 deletion of the entire SUV3 ORF) were assigned to either of these two genes using this strategy.
This suggests that spontaneous [Gly+] suppressors arising in various suv3Δ strains carrying the intronless mitochondrial genome can be attributed to temperature-sensitive partial loss-of-function mutations in the RPO41 and MTF1 genes, encoding the two subunits of the mitochondrial RNA polymerase. The suppression occurs both with partial URA3 disruption and with total KanMX4 deletion alleles of suv3Δ
The Suppressor Mutations Are Novel Single Amino Acid Substitutions in RPO41 and MTF1
We selected the suppressor mutation in the MTF1 gene contained in the SUX3 strain, and two suppressors attributed to mutations in RPO41 (the SUX1 strain and the original SDB9 suppressor) for sequencing analysis. Fragments of genomic DNA corresponding to MTF1 and RPO41 genes were amplified and sequenced using primers described in Materials and Methods. Wild-type isogenic strains W303/A/520 and BWG1 were used as respective controls.
The SUX1 pseudorevertant strain was found to carry a single nucleotide substitution A1628G in the RPO41 ORF (all numbers start from the A in the initiation codon of the ORF), corresponding to the E543G substitution in the translated amino acid sequence. The SDB9 strain, compared with the parental strain BWG1, contained the G2932T substitution in the RPO41 gene, resulting in the V978F mutation in the amino acid sequence. Neither of these mutations has been previously described, they are both located in regions of the Rpo41p sequence displaying homology to the T3 and T7 phage RNA polymerases (Masters et al., 1987).
The MTF1 gene in the SUX3 pseudorevertant, compared with the parental W303/A/520 strain contains a single mutation, C908T, resulting in a P303L substitution in the C-terminal region of the Mtf1p protein. The majority of the temperature-sensitive mutations in the MTF1 gene known so far were located within or close to the regions displaying homology to the σ family of bacterial transcription factors (Shadel and Clayton, 1995; Cliften et al., 1997). Deletion of the C-terminal domain, beyond residue 291, resulted in a temperature-sensitive petite phenotype (Shadel and Clayton, 1995), corresponding to the effect of the P303L substitution observed in the DSW3-12/A spore.
Mutations in the RPO41 and MTF1 Genes Partially Suppressing the suv3Δ [Gly–] Phenotype Decrease the Transcription Rate of the Mitochondrial RNA Polymerase
Mutations in the mitochondrial RNA polymerase genes resulting in a similar, temperature-sensitive petite phenotype described previously (Shadel and Clayton, 1995; Cliften et al., 1997, 2000; Karlok et al., 2002; Matsunaga and Jaehning, 2004) resulted in a general slowing of mitochondrial transcription and a decrease in the abundance of mature RNAs. As the phenotype of the suv3Δ mutation involves dysfunction of mitochondrial RNA degradation and turnover (Golik et al., 1995; Stepien et al., 1995; Dziembowski et al., 2003), it was tempting to speculate that the suppressor mutations we discovered acted through lowering the transcription rate and reducing the RNA content in the organelle.
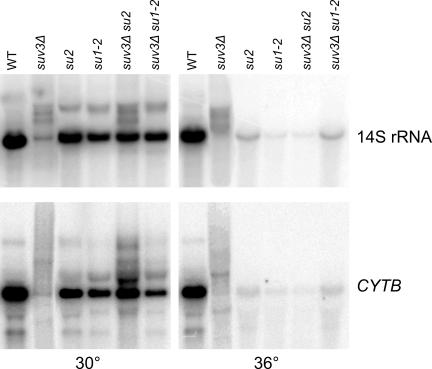
To verify this hypothesis, first we measured the steadystate levels of selected mitochondrial RNA transcripts by Northern hybridization. RNA obtained from purified mitochondria from the wild-type W303/A/520 (WT), suv3Δ (WSU0 Δi), su2 mutant in the MTF1 gene (DSW3-12/A), su1-2 mutant in the RPO41 gene (DSW1–16/C), and pseudorevertant suv3Δ su2 (SUX3) and suv3Δ su1-2 (SUX1) strains, grown at normal (30°C) and elevated (36°C) temperatures was separated on an agarose-formaldehyde gel and hybridized to 14S (SSU) rRNA and CYTB probes. The results are shown in Figure 5. As expected, the suv3Δ strain displays severe RNA processing abnormalities, resulting in very low levels of mature transcripts and accumulation of precursor species, including undefined high-molecular-weight smears as well as precursor bands. Strains carrying the suppressor mutations in MTF1 (su2) and RPO41 (su1-2) in the context of the wild-type SUV3 allele exhibit a marked decrease in the steady-state levels of 14S and CYTB mature transcripts (to ∼33 and 20% of the wild-type level, respectively). In the pseudorevertant strains combining the suv3Δ deletion with the suppressor mutations, the steady-state levels of mature transcripts are restored to the levels similar to those observed in strains carrying the suppressor mutations alone, thus explaining how these mutations can partially restore respiratory competence in the suv3Δ background. Some accumulation of precursors is still observed, in particular in the suv3Δ su2 strain, but the proportion of mature transcript to precursor is much more favorable than in the suv3Δ strain.
Figure 5.
Northern blot analysis of steady-state levels of mitochondrial transcripts of the 14S rRNA and CYTB genes in wild-type W303/A/520 (WT), suv3Δ (WSU0 Δi), su2 mutant in the MTF1 gene (DSW3-12/A), su1-2 mutant in the RPO41 gene (DSW1-16/C), and pseudorevertant suv3Δ su2 (SUX3) and suv3Δ su1-2 (SUX1) strains. RNA was isolated from purified mitochondria, blotted as described in Materials and Methods, and hybridized with the 14S or CYTB oligonucleotide probes. Protein content of each original mitochondrial preparation was used to normalize RNA loading in each lane.
At the elevated temperature the two mitochondrial transcripts in all strains carrying the suppressor mutations were present at extremely low levels, while in the wild-type control their level was still normal. These results correlate with the previously observed phenotype of slower growth on respiratory substrates at normal temperature, and complete respiratory-deficiency at the elevated temperature, discussed above. Interestingly, the effect of the su2 mutation in the MTF1 gene on mitochondrial transcription is less pronounced than that of the su1 mutation in the RPO41 gene, which correlates with the increased accumulation of residual RNA precursors in the suv3Δ su2 strain compared with the suv3Δ su1 strain and slightly slower respiratory growth.
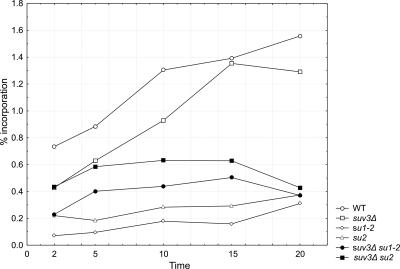
To verify whether the lower abundance of the mitochondrial transcripts observed in the suppressor mutant strains was indeed related to a decrease in transcription, we measured the mitochondrial transcription rates of the mutant strains using an in organello [α-32P]UTP incorporation assay (Krause and Dieckmann, 2004). Mitochondria from wild-type W303/A/520 (WT), suv3Δ (WSU0 Δi), su2 mutant in the MTF1 gene (DSW3-12/A), su1-2 mutant in the RPO41 gene (DSW1-16/C), and pseudorevertant suv3Δ su2 (SUX3) and suv3Δ su1-2 (SUX1) strains, grown at a normal temperature to mid-log phase, were purified by differential centrifugation and incubated in transcription buffer containing [α-32P]UTP and an RNase inhibitor (see Materials and Methods). The results, shown in Figure 6, demonstrate that although the wild-type strain shows typical rapid kinetics of the labeled precursor incorporation, the incorporation in the su mutant strains is much slower and never reaches wild-type levels, even upon prolonged incubation. Transcription in the suv3Δ strain is slightly lower than in the wild type, which may be related to the increased frequency of rho–/rho0 petites. Pseudorevertant strains suv3Δ su2 and suv3Δ su1-2 show slight improvement over the strains containing the suppressor mutations alone, which may be explained by the reduced RNA degradation activity in suv3Δ mitochondria. At 10 min of incubation, the incorporation in su1-2 (rpo41) and su2 (mtf1) mutants is ∼10 and 18% of the wild-type value, respectively.
Figure 6.
In organello transcription assay measuring labeled [α-32P]UTP incorporation into purified mitochondria of the wild-type W303/A/520 (WT), suv3Δ (WSU0 Δi), su2 mutant in the MTF1 gene (DSW3-12/A), su1-2 mutant in the RPO41 gene (DSW1-16/C), and pseudorevertant suv3Δ su2 (SUX3) and suv3Δ su1-2 (SUX1) strains. Typical results of one from at least three independent experiments are shown for each strain as percent incorporation.
We observed the same trend in several independent repetitions of the experiment, although absolute incorporation values were variable and the incorporation levels of the mutant strains were close to the sensitivity limit of the assay, which made a more rigorous statistical treatment of the quantitative results impossible. Positive incorporation in the mutant strains becomes more obvious when more than 5 μg mitochondrial preparation per reaction is used in the experiment; in such case, however, the wild-type preparation becomes overloaded and gives maximum signal already at the first time point.
These results demonstrate that the suppressor mutations in the RPO41 and MTF1 genes do indeed act by drastically slowing down the transcription rate in mitochondria, as demonstrated by the in organello incorporation assay. The effect on the steady-state level of mature RNAs seems slightly less pronounced, the two methods are, however, not directly comparable. Besides, more factors than just the transcription rate influence the steady-state level of transcripts in vivo which might explain the more moderate effect seen in the Northern hybridization assay. Although the effect of the suppressor mutations on transcription might seem drastic, respiratory competence of yeast mutants having <10% of the wild-type level of a mature mitochondrial transcript has been reported previously (Schmidt et al., 1998). In the suv3Δ background, decreased transcription rates result in partial restoration of mature RNAs and decrease in the accumulation of precursors, so that the mature transcripts become the dominant form again.
DISCUSSION
The RNA helicase encoded by the SUV3 gene is a critical component of the yeast mitochondrial degradosome (Dziembowski et al., 2003) and is essential for the expression and maintenance of the mitochondrial genome. A prominent feature of SUV3-deficient yeast strains is the accumulation of aberrant RNA species. Some of them can be attributed to unprocessed intronic sequences (Stepien et al., 1995), but the abnormal accumulation of high-molecular-weight mitochondrial RNAs is also observed in the intronless strain (Dziembowski et al., 1998, 2003). Because some mature RNAs are still present in intronless mitochondria in degradosome-deficient strains, it has been suggested that impaired degradation of aberrant and unprocessed transcripts, rather than a block in the process of RNA maturation, is responsible for the molecular phenotype of SUV3 or DSS1/MSU1 deletion. In this model, the mitochondrial degradosome is responsible for the RNA surveillance function in the organelle.
The burden of aberrant and unprocessed RNA species could have a detrimental effect on the functioning of the mitochondrial genetic system. Accumulation of undegraded RNAs could disrupt the assembly of mitochondrial RNP complexes, and perturb the ribonucleotide pools. In particular, assembly and functioning of the mitochondrial ribosomes could be affected, because the accumulation of rRNA and VAR1 precursors has been observed in degradosome-deficient strains (Dziembowski et al., 1998, 2003). Perturbations in mitochondrial translation are known to affect the stability of mitochondrial DNA (Myers et al., 1985), which would explain the elevated frequency of mitochondrial petites observed in suv3Δ strains (Stepien et al., 1992, 1995; Golik et al., 1995). Defects in the quality control of ribosomal RNAs leading to defects in ribosome maturation were found to cause lethality in Escherichia coli strains devoid of the two major exoribonucleases, RNase R and polynucleotide phosphorylase (Cheng and Deutscher, 2003). Although the polynucleotide phosphorylase has been found in human mitochondria (Piwowarski et al., 2003), it is conspicuously absent from the mitochondria of S. cerevisiae. The mtEXO complex is therefore the sole major exoribonuclease of yeast mitochondria and its inactivation could cause disturbances similar in extent to those observed in bacterial cells lacking the two major RNase enzymes.
In this study, we demonstrated that spontaneous suppressor mutations partially rescuing the functioning of the mitochondrial genetic system in degradosome-deficient suv3Δ strains occur in the MTF1 (YMR228W) and RPO41 (YFL036W) genes encoding the two subunits of the mitochondrial RNA polymerase (Kelly et al., 1986; Masters et al., 1987; Jang and Jaehning, 1991; Ulery and Jaehning, 1994). They are temperature-sensitive, recessive, partial loss-of-function (hypomorphic) mutations that reduce the transcription rate at normal temperature and nearly abolish the mitochondrial RNA polymerase function at elevated temperature. At 30°C, mitochondrial transcription in mutant strains is <18% of wild-type level (as determined by nucleotide incorporation assay in organello), which also leads to reduced levels of mature transcripts (as detected by Northern blotting). It is also worth noting that the system we describe allows for positive selection of hypomorphic mutants in the RNA polymerase genes, which could be beneficial for studying the structure-function relationships in the RNAP protein subunits.
Partial rescue of the mitochondrial genetic function in the suppressor strains is linked to this decrease of mitochondrial transcription. Cells deficient in the mtEXO function cannot provide sufficient RNA degradation activity to cope with the production of unprocessed and aberrant RNAs associated with normal transcription rates. The pseudorevertant strains, with their reduced transcription rate, produce less RNA, including less unprocessed and/or aberrant transcripts, so that the mitochondrial genetic system can continue to function, albeit at a visibly lower level that translates to slower respiratory growth. In this model, decrease of the transcription rate caused by the suppressor mutations restores, at least partially, the balance between RNA synthesis and degradation disrupted by the genetic inactivation of the degradosome function. On the molecular level, this is manifested by shifting of the mature RNA/precursor ratios toward normal values, allowing sufficient expression of mitochondrially encoded genes. Although some overaccumulation of precursors is still observed in degradosome-deficient strains carrying the suppressor mutations, mature RNAs are the dominant form, like in wild-type mitochondria and unlike in the degradosome-deficient organelles.
It is possible that with the diminished transcriptional activity of suppressor mutants, the aberrant RNA species accumulate at a level that is so low that even without their removal, normally ensured by the degradosome, they do not interfere with mitochondrial function. Most likely, however, some residual exoribonuclease activity is responsible for the degradation of low levels of transcripts in suppressor strains. This activity could be provided by the RNase subunit of the mitochondrial degradosome encoded by the DSS1/MSU1 gene, probably in association with another RNA helicase. An obvious candidate would be the Mss1p mitochondrial RNA helicase (Seraphin et al., 1989), which can partially rescue mitochondrial gene expression in suv3Δ strains when overexpressed from a multicopy plasmid (Minczuk et al., 2002).
Another explanation would postulate the existence of another exoribonuclease in yeast mitochondria, which alone cannot provide sufficient degradation activity to provide the RNA turnover and surveillance function with the normal transcript levels produced by the wild-type RNA polymerase, but it can nevertheless cope with the severely reduced RNA quantities in the suppressor mutants. In E. coli, there are eight 3′-to-5′ exoribonucleases (Deutscher and Li, 2001). Three of these, RNase II, RNase R, and polynucleotide phosphorylase, provide nonspecific processive activity required for the degradation of transcripts (Zuo and Deutscher, 2001; Cheng and Deutscher, 2005). The RNase component of the mitochondrial degradosome encoded by the DSS1/MSU1 gene remains, however, the only yeast processive 3′-to-5′ exoribonuclease found in mitochondria. The protein encoded by the NUC1 gene (Zassenhaus et al., 1988), once thought to be the main mitochondrial nuclease, was found not to be involved in RNA degradation (Dziembowski et al., 1998). The Ynt20p (Rex2p) 3′-to-5′ mitochondrial ribonuclease (Hanekamp and Thorsness, 1999) has a distributive activity, similar to the bacterial oligoribonuclease (Zuo and Deutscher, 2001) and is involved in the maturation of small RNAs (van Hoof et al., 2000). The activity of Ynt20p family nucleases seems to be limited to degradation of very short single-stranded oligonucleotides (Nguyen et al., 2000). None of the known proteins can be therefore considered as a candidate for the putative secondary 3′-5′ RNA degradation activity in yeast mitochondria that could supplement the mtEXO complex. Although this does not rule out the existence of such activity, no obvious candidate genes are apparent in the genome of S. cerevisiae. Alternatively, the 5′ end-directed degradation activity mediated by the product of the PET127 gene (Krause and Dieckmann, 2004) could compensate for the lack of the 3′-to-5′ degradosome activity.
One of the particular characteristics of the mitochondrial genetic system is the close association between different levels of gene expression. RNA processing and translation have been shown to be physically coupled to transcription through a network of protein–protein and protein–RNA interactions (Rodeheffer et al., 2001; Bryan et al., 2002; Krause et al., 2004). Because the mitochondrial degradosome is associated with the ribosome (Dziembowski et al., 2003), it could also be a part of this network, offering another explanation for the genetic interaction between transcription and RNA degradation uncovered in this work.
The results of this study underscore the essential character of RNA degradation in the expression of genetic information. Mitochondria, with their simplified transcriptional control seem to be particularly dependent on posttranscriptional processes, including RNA degradation and surveillance. Our results clearly demonstrate that maintaining the balance between RNA synthesis and degradation is crucial to the functioning of the mitochondrial genetic system. Although the enzymatic machinery of mitochondrial gene expression shows considerable evolutionary divergence among phyla and is very different in higher eukaryotes in comparison with S. cerevisiae, a common theme of very simple transcription and complex posttranscriptional processing of RNAs is shared in mitochondria from yeasts to human. Understanding the mechanisms that maintain balance between the RNA synthesis and degradation is therefore a fundamental area of mitochondrial science.
Acknowledgments
We thank Dr. Judith Jaehning for the gift of plasmids pJJ1148 and pJJ1149 and Dr. Mark Johnston for the gift of the yeast plasmid library constructed by P. Hieter. We are grateful to Prof. Ewa Bartnik for critical reading of the manuscript. This work was supported by The State Committee for Scientific Research, KBN, through The Faculty of Biology, Warsaw University Intramural Grants BW#52/2003 and BW#19/2004.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–08–0796) on December 21, 2005.
References
- Bryan, A. C., Rodeheffer, M. S., Wearn, C. M., and Shadel, G. S. (2002). Sls1p is a membrane-bound regulator of transcription-coupled processes involved in Saccharomyces cerevisiae mitochondrial gene expression. Genetics 160, 75–82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke, D., Dawson, D., and Stearns, T. (2000). Methods in Yeast Genetics, Cold Spring Harbor, NY: Cold Spring Harbor Laboratory Press.
- Carpousis, A. J. (2002). The Escherichia coli RNA degradosome: structure, function and relationship in other ribonucleolytic multienzyme complexes. Biochem. Soc. Trans. 30, 150–155. [PubMed] [Google Scholar]
- Chen, W., Islas-Osuna, M. A., and Dieckmann, C. L. (1999). Suppressor analysis of mutations in the 5′-untranslated region of COB mRNA identifies components of general pathways for mitochondrial mRNA processing and decay in Saccharomyces cerevisiae. Genetics 151, 1315–1325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. F., and Deutscher, M. P. (2003). Quality control of ribosomal RNA mediated by polynucleotide phosphorylase and RNase R. Proc. Natl. Acad. Sci. USA 100, 6388–6393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng, Z. F., and Deutscher, M. P. (2005). An important role for RNase R in mRNA decay. Mol. Cell 17, 313–318. [DOI] [PubMed] [Google Scholar]
- Christianson, T., and Rabinowitz, M. (1983). Identification of multiple transcriptional initiation sites on the yeast mitochondrial genome by in vitro capping with guanylyltransferase. J. Biol. Chem. 258, 14025–14033. [PubMed] [Google Scholar]
- Cliften, P. F., Jang, S. H., and Jaehning, J. A. (2000). Identifying a core RNA polymerase surface critical for interactions with a sigma-like specificity factor. Mol. Cell. Biol. 20, 7013–7023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cliften, P. F., Park, J. Y., Davis, B. P., Jang, S. H., and Jaehning, J. A. (1997). Identification of three regions essential for interaction between a sigma-like factor and core RNA polymerase. Genes Dev. 11, 2897–2909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deutscher, M. P., and Li, Z. (2001). Exoribonucleases and their multiple roles in RNA metabolism. Prog. Nucleic Acid Res. Mol. Biol. 66, 67–105. [DOI] [PubMed] [Google Scholar]
- Dmochowska, A., Golik, P., and Stepien, P. P. (1995). The novel nuclear gene DSS-1 of Saccharomyces cerevisiae is necessary for mitochondrial biogenesis. Curr. Genet. 28, 108–112. [DOI] [PubMed] [Google Scholar]
- Dujardin, G., Pajot, P., Groudinsky, O., and Slonimski, P. P. (1980). Long range control circuits within mitochondria and between nucleus and mitochondria. I. Methodology and phenomenology of suppressors. Mol. Gen. Genet. 179, 469–482. [DOI] [PubMed] [Google Scholar]
- Dziembowski, A., Malewicz, M., Minczuk, M., Golik, P., Dmochowska, A., and Stepien, P. P. (1998). The yeast nuclear gene DSS1, which codes for a putative RNase II, is necessary for the function of the mitochondrial degradosome in processing and turnover of RNA. Mol. Gen. Genet. 260, 108–114. [DOI] [PubMed] [Google Scholar]
- Dziembowski, A., Piwowarski, J., Hoser, R., Minczuk, M., Dmochowska, A., Siep, M., van der Spek, H., Grivell, L., and Stepien, P. P. (2003). The yeast mitochondrial degradosome. Its composition, interplay between RNA helicase and RNase activities and the role in mitochondrial RNA metabolism. J. Biol. Chem. 278, 1603–1611. [DOI] [PubMed] [Google Scholar]
- Foury, F., Roganti, T., Lecrenier, N., and Purnelle, B. (1998). The complete sequence of the mitochondrial genome of Saccharomyces cerevisiae. FEBS Lett. 440, 325–331. [DOI] [PubMed] [Google Scholar]
- Gagliardi, D., Stepien, P. P., Temperley, R. J., Lightowlers, R. N., and Chrzanowska-Lightowlers, Z. M. (2004). Messenger RNA stability in mitochondria: different means to an end. Trends Genet. 20, 260–267. [DOI] [PubMed] [Google Scholar]
- Giaever, G., et al. (2002). Functional profiling of the Saccharomyces cerevisiae genome. Nature 418, 387–391. [DOI] [PubMed] [Google Scholar]
- Gietz, R. D., and Sugino, A. (1988). New yeast-Escherichia coli shuttle vectors constructed with in vitro mutagenized yeast genes lacking six-base pair restriction sites. Gene 74, 527–534. [DOI] [PubMed] [Google Scholar]
- Gietz, R. D., and Woods, R. A. (2002). Transformation of yeast by lithium acetate/single-stranded carrier DNA/polyethylene glycol method. Methods Enzymol. 350, 87–96. [DOI] [PubMed] [Google Scholar]
- Golik, P., Szczepanek, T., Bartnik, E., Stepien, P. P., and Lazowska, J. (1995). The S. cerevisiae nuclear gene SUV3 encoding a putative RNA helicase is necessary for the stability of mitochondrial transcripts containing multiple introns. Curr. Genet. 28, 217–224. [DOI] [PubMed] [Google Scholar]
- Groudinsky, O., Dujardin, G., and Slonimski, P. P. (1981). Long range control circuits within mitochondria and between nucleus and mitochondria. II. Genetic and biochemical analyses of suppressors which selectively alleviate the mitochondrial intron mutations. Mol. Gen. Genet. 184, 493–503. [DOI] [PubMed] [Google Scholar]
- Hanekamp, T., and Thorsness, P. E. (1999). YNT20, a bypass suppressor of yme1 yme2, encodes a putative 3′-5′ exonuclease localized in mitochondria of Saccharomyces cerevisiae. Curr. Genet. 34, 438–448. [DOI] [PubMed] [Google Scholar]
- Hoffman, C. S., and Winston, F. (1987). A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57, 267–272. [DOI] [PubMed] [Google Scholar]
- Jang, S. H., and Jaehning, J. A. (1991). The yeast mitochondrial RNA polymerase specificity factor, MTF1, is similar to bacterial sigma factors. J. Biol. Chem. 266, 22671–22677. [PubMed] [Google Scholar]
- Karlok, M. A., Jang, S. H., and Jaehning, J. A. (2002). Mutations in the yeast mitochondrial RNA polymerase specificity factor, Mtf1, verify an essential role in promoter utilization. J. Biol. Chem. 277, 28143–28149. [DOI] [PubMed] [Google Scholar]
- Kelly, J. L., Greenleaf, A. L., and Lehman, I. R. (1986). Isolation of the nuclear gene encoding a subunit of the yeast mitochondrial RNA polymerase. J. Biol. Chem. 261, 10348–10351. [PubMed] [Google Scholar]
- Krause, K., and Dieckmann, C. L. (2004). Analysis of transcription asymmetries along the tRNAE-COB operon: evidence for transcription attenuation and rapid RNA degradation between coding sequences. Nucleic Acids Res. 32, 6276–6283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krause, K., Lopes de Souza, R., Roberts, D. G., and Dieckmann, C. L. (2004). The mitochondrial message-specific mRNA protectors Cbp1 and Pet309 are associated in a high-molecular weight complex. Mol. Biol. Cell 15, 2674–2683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mangus, D. A., Jang, S. H., and Jaehning, J. A. (1994). Release of the yeast mitochondrial RNA polymerase specificity factor from transcription complexes. J. Biol. Chem. 269, 26568–26574. [PubMed] [Google Scholar]
- Margossian, S. P., and Butow, R. A. (1996). RNA turnover and the control of mitochondrial gene expression. Trends Biochem. Sci. 21, 392–396. [PubMed] [Google Scholar]
- Margossian, S. P., Li, H., Zassenhaus, H. P., and Butow, R. A. (1996). The DExH box protein Suv3p is a component of a yeast mitochondrial 3′-to-5′ exoribonuclease that suppresses group I intron toxicity. Cell 84, 199–209. [DOI] [PubMed] [Google Scholar]
- Masters, B. S., Stohl, L. L., and Clayton, D. A. (1987). Yeast mitochondrial RNA polymerase is homologous to those encoded by bacteriophages T3 and T7. Cell 51, 89–99. [DOI] [PubMed] [Google Scholar]
- Matsunaga, M., and Jaehning, J. A. (2004). A mutation in the yeast mitochondrial core RNA polymerase, Rpo41, confers defects in both specificity factor interaction and promoter utilization. J. Biol. Chem. 279, 2012–2019. [DOI] [PubMed] [Google Scholar]
- Minczuk, M., Dmochowska, A., Palczewska, M., and Stepien, P. P. (2002). Overexpressed yeast mitochondrial putative RNA helicase Mss116 partially restores proper mtRNA metabolism in strains lacking the Suv3 mtRNA helicase. Yeast 19, 1285–1293. [DOI] [PubMed] [Google Scholar]
- Myers, A. M., Pape, L. K., and Tzagoloff, A. (1985). Mitochondrial protein synthesis is required for maintenance of intact mitochondrial genomes in Saccharomyces cerevisiae. EMBO J. 4, 2087–2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nguyen, L. H., Erzberger, J. P., Root, J., and Wilson, D. M., 3rd. (2000). The human homolog of Escherichia coli Orn degrades small single-stranded RNA and DNA oligomers. J. Biol. Chem. 275, 25900–25906. [DOI] [PubMed] [Google Scholar]
- Osinga, K. A., De Haan, M., Christianson, T., and Tabak, H. F. (1982). A nonanucleotide sequence involved in promotion of ribosomal RNA synthesis and RNA priming of DNA replication in yeast mitochondria. Nucleic Acids Res. 10, 7993–8006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piwowarski, J., Grzechnik, P., Dziembowski, A., Dmochowska, A., Minczuk, M., and Stepien, P. P. (2003). Human polynucleotide phosphorylase, hPN-Pase, is localized in mitochondria. J. Mol. Biol. 329, 853–857. [DOI] [PubMed] [Google Scholar]
- Rodeheffer, M. S., Boone, B. E., Bryan, A. C., and Shadel, G. S. (2001). Nam1p, a protein involved in RNA processing and translation, is coupled to transcription through an interaction with yeast mitochondrial RNA polymerase. J. Biol. Chem. 276, 8616–8622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schafer, B. (2005). RNA maturation in mitochondria of S. cerevisiae and S. pombe. Gene 354, 80–85. [DOI] [PubMed] [Google Scholar]
- Schinkel, A. H., Koerkamp, M. J., Touw, E. P., and Tabak, H. F. (1987). Specificity factor of yeast mitochondrial RNA polymerase. Purification and interaction with core RNA polymerase. J. Biol. Chem. 262, 12785–12791. [PubMed] [Google Scholar]
- Schmidt, U., Maue, I., Lehmann, K., Belcher, S. M., Stahl, U., and Perlman, P. S. (1998). Mutant alleles of the MRS2 gene of yeast nuclear DNA suppress mutations in the catalytic core of a mitochondrial group II intron. J. Mol. Biol. 282, 525–541. [DOI] [PubMed] [Google Scholar]
- Seraphin, B., Boulet, A., Simon, M., and Faye, G. (1987). Construction of a yeast strain devoid of mitochondrial introns and its use to screen nuclear genes involved in mitochondrial splicing. Proc. Natl. Acad. Sci. USA 84, 6810–6814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seraphin, B., Simon, M., Boulet, A., and Faye, G. (1989). Mitochondrial splicing requires a protein from a novel helicase family. Nature 337, 84–87. [DOI] [PubMed] [Google Scholar]
- Shadel, G. S., and Clayton, D. A. (1995). A Saccharomyces cerevisiae mitochondrial transcription factor, sc-mtTFB, shares features with sigma factors but is functionally distinct. Mol. Cell. Biol. 15, 2101–2108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stepien, P. P., Kokot, L., Leski, T., and Bartnik, E. (1995). The suv3 nuclear gene product is required for the in vivo processing of the yeast mitochondrial 21s rRNA transcripts containing the r1 intron. Curr. Genet. 27, 234–238. [DOI] [PubMed] [Google Scholar]
- Stepien, P. P., Margossian, S. P., Landsman, D., and Butow, R. A. (1992). The yeast nuclear gene suv3 affecting mitochondrial post-transcriptional processes encodes a putative ATP-dependent RNA helicase. Proc. Natl. Acad. Sci. USA 89, 6813–6817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomecki, R., Dmochowska, A., Gewartowski, K., Dziembowski, A., and Stepien, P. P. (2004). Identification of a novel human nuclear-encoded mitochondrial poly(A) polymerase. Nucleic Acids Res. 32, 6001–6014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzagoloff, A., and Myers, A. M. (1986). Genetics of mitochondrial biogenesis. Annu. Rev. Biochem. 55, 249–285. [DOI] [PubMed] [Google Scholar]
- Ulery, T. L., and Jaehning, J. A. (1994). MTF1, encoding the yeast mitochondrial RNA polymerase specificity factor, is located on chromosome XIII. Yeast 10, 839–841. [DOI] [PubMed] [Google Scholar]
- van Hoof, A., Lennertz, P., and Parker, R. (2000). Three conserved members of the RNase D family have unique and overlapping functions in the processing of 5S, 5.8S, U4, U5, RNase MRP and RNase P RNAs in yeast. EMBO J. 19, 1357–1365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasudevan, S., and Peltz, S. W. (2003). Nuclear mRNA surveillance. Curr. Opin. Cell Biol. 15, 332–337. [DOI] [PubMed] [Google Scholar]
- Wegierski, T., Dmochowska, A., Jablonowska, A., Dziembowski, A., Bartnik, E., and Stepien, P. P. (1998). Yeast nuclear PET127 gene can suppress deletions of the SUV3 or DSS1 genes: an indication of a functional interaction between 3′ and 5′ ends of mitochondrial mRNAs. Acta Biochim. Pol. 45, 935–940. [PubMed] [Google Scholar]
- Wiesenberger, G., and Fox, T. D. (1997). Pet127p, a membrane-associated protein involved in stability and processing of Saccharomyces cerevisiae mitochondrial RNAs. Mol. Cell. Biol. 17, 2816–2824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winzeler, E. A., et al. (1999). Functional characterization of the S. cerevisiae genome by gene deletion and parallel analysis. Science 285, 901–906. [DOI] [PubMed] [Google Scholar]
- Zassenhaus, H. P., Hofmann, T. J., Uthayashanker, R., Vincent, R. D., and Zona, M. (1988). Construction of a yeast mutant lacking the mitochondrial nuclease. Nucleic Acids Res. 16, 3283–3296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuo, Y., and Deutscher, M. P. (2001). Exoribonuclease superfamilies: structural analysis and phylogenetic distribution. Nucleic Acids Res. 29, 1017–1026. [DOI] [PMC free article] [PubMed] [Google Scholar]