Abstract
Receptor-mediated internalization to the endoplasmic reticulum (ER) and subsequent retro-translocation to the cytosol are essential sequential processes required for the productive intoxication of susceptible mammalian cells by Shiga-like toxin-1 (SLTx). Recently, it has been proposed that the observed association of certain ER-directed toxins and viruses with detergent-resistant membranes (DRM) may provide a general mechanism for their retrograde transport to endoplasmic reticulum (ER). Here, we show that DRM recruitment of SLTx bound to its globotriosylceramide (Gb3) receptor is mediated by the availability of other glycosphingolipids. Reduction in glucosylceramide (GlcCer) levels led to complete protection against SLTx and a reduced cell surface association of bound toxin with DRM. This reduction still allowed efficient binding and transport of the toxin to the ER. However, toxin sequestration within DRM of the ER was abolished under reduced GlcCer conditions, suggesting that an association of toxin with lipid microdomains or rafts in the ER (where these are defined by detergent insolubility) is essential for a later step leading to or involving retro-translocation of SLTx across the ER membrane. In support of this, we show that a number of ER residents, proteins intimately involved in the process of ER dislocation of misfolded proteins, are present in DRM.
INTRODUCTION
Shiga-like toxin 1 (SLTx) is a potent ribosome-inactivating protein produced by certain enterohemorrhagic strains of Escherichia coli and is virtually identical to Shigella toxin (STx). It consists of a single A-chain possessing rRNA-specific N-glycosidase activity (Endo et al., 1988) and five noncovalently associated B-subunits (SLTxB) that are responsible for binding and uptake into target cells (Pudymaitis et al., 1991; Sandvig et al., 1994; Ling et al., 1998). To inactivate ribosomes, SLTx (or at least the enzymatic A subunit) must traverse an internal membrane, thought to be the endoplasmic reticulum (ER) membrane. This is also the retro-translocation site for internalized STx, cholera toxin (CTx), ricin, E. coli heat-labile enterotoxin, pertussis toxin, and Pseudomonas exotoxin A (Lencer and Tsai, 2003; Lord et al., 2003; Roberts and Smith, 2004; Sandvig et al., 2004) whose substrates also reside within the cytosol. Although the details of toxin translocation remain sketchy, it is thought that these proteins can co-opt ER components and exploit the ER retro-translocation machinery normally used in the perception and dislocation of newly made but misfolded or orphan polypeptides in readiness for their destruction by proteasomes. To be effective, however, the toxins must somehow uncouple from downstream cytosolic steps to avoid being completely degraded (Hazes and Read, 1997; Deeks et al., 2002; Rodighiero et al., 2002).
To reach the ER, SLTx must first bind, through the B subunits, to its globotriaosylceramide (Gb3/CD77) receptors at the plasma membrane (Jacewicz et al., 1986). Internalization of the toxin is known to proceed via the endosomal system and the trans-Golgi network in a process that by-passes late endosomes (Mallard et al., 1998; Mallard et al., 2002). This is followed by coatomer protein 1-independent transport through the Golgi to the ER (Girod et al., 1999; White et al., 1999). Although the extent and identity of cellular proteins trafficking from the plasma membrane to the early Golgi and ER are not fully known, the retrograde trafficking of some glycosphingolipids (GSL) to the Golgi has been recognized (Puri et al., 2001). It has recently been proposed that the retrograde routing of GSL-binding toxins to the ER involves association of the toxin/GSL complex with lipid microdomains (Fujinaga et al., 2003), often referred to as lipid rafts that are enriched in certain GSL, specific proteins, and cholesterol (Simons and Ikonen, 1997; Brown and London, 2000). These are isolated on the basis of detergent resistance, and the resulting detergent-resistant membranes (DRM) are interpreted to represent lipid microdomains in the original membrane. Indeed, there is a strong correlation between the ER routing of the B-chain of the related STx and its association with DRM in toxin-sensitive HeLa cells. By contrast, in a macrophage cell line in which DRM association of STx B was not observed, the toxin was directed to lysosomes, and the cells survived (Falguieres et al., 2001).
Recently, it has been shown that the normal retrograde transport of an internalized GSL, lactosylceramide (LacCer), to the Golgi can be altered dramatically by chemical modulation of endogenous glucosylceramide (GlcCer) levels (Sillence et al., 2002). Indeed, treatment of RAW macrophages with the imino sugar N-butyldeoxygalactonojirimycin (NB-DGJ), a specific inhibitor of ceramide glucosyltransferase (Andersson et al., 2000), resulted in lysosomal targeting of LacCer (Sillence et al., 2002). A question arising from this is whether modulation of cellular GlcCer levels also affects the trafficking and cytotoxicity of SLTx.
In the present study, we have investigated the effect of NB-DGJ on the intracellular uptake and cytotoxicity of SLTx. We show that NB-DGJ completely protected both Vero and HeLa cells from SLTx intoxication. Furthermore, we have shown that the affinity of toxin for DRM was modulated by the presence of GlcCer, such that its partitioning into DRM was lowered at the plasma membrane and abolished within the ER upon treatment with the inhibitor. Membrane binding and subsequent retrograde transport of toxin to the ER was unaffected by NB-DGJ treatment. This result led us to propose that SLTx requires an association with lipid rafts within the ER membrane for efficient retro-translocation to the cytosol. In support of this, we have demonstrated that certain ER-resident proteins, which are intimately involved in the retro-translocation process, are themselves sequestered into DRM.
MATERIALS AND METHODS
Sources
NB-DGJ was purchased from Calbiochem (San Diego, CA). Antibodies were kindly provided against calnexin, ribophorins, and Sec61β by Steve High (University of Manchester, Manchester, United Kingdom), Derlin-1 and VIMP by Tom Rapoport (Harvard University, Cambridge, MA), and SLTx by Arthur Donohue-Rolfe (Tufts University, Boston, MA). Antibody against protein disulfide isomerase (PDI) was purchased from Stressgen Biotechnologies (Victoria, British Columbia, Canada), and antibody against transferrin receptor was purchased from Zymed (South San Francisco, CA). Radioisotopes were from GE Healthcare (Little Chalfont, Buckinghamshire, United Kingdom). Cross-linkers and IODO-BEADS iodination reagent were obtained from Pierce Chemical (Cheshire, United Kingdom). Cyclosporin A came from Sigma (Poole, Dorset, United Kingdom). Glucosylsphingosine came from Matreya (Universal Biologicals, Stroud, United Kingdom).
Cell Lines
All cell lines were maintained in RPMI 1640 medium containing 2 mM l-glutamine, 100 U/ml penicillin, 100 μg/ml streptomycin (Invitrogen, Carlsbad, CA), and 5% fetal calf serum (Labtech, Ringmer, United Kingdom).
Cytotoxicity Assays
Cells were seeded at 2.5 × 104 cells/well into flat-bottomed 96-well plates and grown overnight. Cells were then overlaid with medium containing appropriate concentrations of SLTx for 4 h. Remaining cellular protein synthesis after toxin treatment was determined as the incorporation of [35S]methionine into total protein as described previously (Deeks et al., 2002; Smith et al., 2003)
Biochemistry, Binding, and Internalization Analysis
Protein iodination, Scatchard analysis, and glycosylation analysis were performed as described previously (Johannes et al., 1997; Mallard et al., 1998; Spooner et al., 2004).
Preparation and Analysis of DRM from HeLa and L3/bcl-2 Cells
DRM were prepared as published previously (Benting et al., 1999; Falguieres et al., 2001). Briefly, the required amount of cells for 300 μg of total protein was washed once with phosphate-buffered saline (PBS) and resuspended in 0.2 ml of 2× TNE (1× TNE: 25 mM Tris-HCl,pH 7.4, 150 mM NaCl, 2.5 mM EDTA, and a mixture of protease inhibitors containing phenylmethylsulfonyl fluoride, leupeptin, chymostatin, pepstatin, antipain, and aprotinin). All steps were performed on ice. Cells were lysed by passage through a ball homogenizer (20 times). The postnuclear supernatant (PNS) was collected after a spin at 4°C, 3000 × g for 10 min; the 0.2-ml volume was added to an equal volume of 2% Triton X-100 (Tx-100), and the mixture was incubated on ice for 30 min. OptiPrep (Sigma) was added to a final concentration of 40%. The solutions were overlaid with 2.3 and 0.8 ml of 30 and 5% OptiPrep in TNE, respectively, and spun at 4°C, 28,000 rpm, for 4 h (SW41 rotor; Beckman Coulter, Fullerton, CA). The gradient was fractionated manually from the top. DRM were found at the 5/30% interface. To analyze the fractions, Western blotting and dot blot analysis were performed according to standard procedures.
Glycolipid Extraction from L3/bcl-2 Cells and Toxin Overlay
Lipid extraction was carried out according to the published method (Bligh and Dyer, 1959), with the previously described modifications (Falguieres et al., 2001), as described above. After DRM gradient fractionation, the isolated neutral glycolipids were spotted on high-performance thin layer chromatography (TLC) plates (Merck, Darmstadt, Germany) and separated with chloroform/methanol/water (65:25:4). Dried plates were soaked in 0.1% polyisobutylmethacrylate in hexane and floated for 1 h in blocking solution, followed by incubation with 20 nM SLTxB, primary polyclonal anti-SLTxB, and secondary horseradish peroxidase, or alkaline phosphatase-coupled anti-rabbit antibodies. Reactive bands were revealed with the use of enhanced chemifluorescence (GE Healthcare) and PhosphorImager.
Isolation of DRM for Lipid Analysis
DRM were purified as published previously (Lisanti et al., 1995). Briefly, tissues were homogenized with 10 strokes of a Dounce homogenizer in PBS. The PNS was collected after a spin at 4°C, 3000 × g for 10 min, the volume was added to an equal volume of 1% Tx-100, and the mixture was incubated on ice for 30 min. The solution made up to 40% sucrose and transferred to a Beckman Ultraclear 14 × 89-mm tube on ice, and a discontinuous gradient of 5 ml of 30% sucrose and 3 ml of 5% sucrose was layered on top. Samples were ultracentrifuged in a SW41 rotor at 100,000 × g for 16 h at 4°C, and 2-ml fractions were manually collected.
Purification and Preparation of GSL for HPLC
GlcCer was purified on silicic acid columns, washed with 2 ml of CHCl3 and 2 ml of CHCl3/MeOH (98:2). GlcCer was eluted with 2 ml of CHCl3/MeOH (97:3), 2 ml of CHCl3/MeOH (96:4), 2 ml of CHCl3/MeOH (95:5), and 2 ml of CHCl3/MeOH (94:6), and dried down and resuspended in 15 μl of 50 mM sodium acetate buffer, pH 5, with 0.1% Triton and 0.25% taurocholate. Then, 0.4 U of purified glucocerebrosidase (cerezyme) was added and incubated at 37°C for 18 h. Samples were derivatized using the same method as for the GSL. Other GSL were extracted by the addition of 3.2 vol of CHCl3/MeOH (1:2.2) for 10 min at room temperature, and the phases were split by the addition of 1 vol of CHCl3 and 1 vol of H2O (Bligh and Dyer, 1959). This quick extraction procedure gave similar recoveries of GSL to other commonly used methods of lipid extraction (van Echten et al., 1990; Miller-Podraza et al., 1992). Upper phase GSL were then recovered by SePak C18 columns (Sillence et al., 2000). Briefly, C18 SepPak columns (Waters, Milford, MA) were washed with 1 ml of MeOH and 1 ml of water. The CHCl3 phase was dried down and loaded in 50 μl of CHCl3/MeOH (1:3). The corresponding aqueous phase was loaded onto the columns and washed with 1 ml of water five times. GSL were then eluted with 1 ml of CHCl3/MeOH (1:3) five times and 1 ml of MeOH, and the samples were dried under nitrogen. At this point, the cholesterol and GSLs were quantified as described below. For mass spectrometry, the samples were saponified by the addition of 1 ml of CHCl3 and 1 ml of 0.2 M NaOH in methanol and incubated overnight at 37°C and further purified by silicic acid chromatography (Vance and Sweeley, 1967). Silicic acid columns were pre-equilibrated in CHCl3, and the samples were loaded onto the column and washed with 5 ml of CHCl3. Neutral GSL were eluted with 6 ml of acetone/MeOH (9:1). Gangliosides were eluted with 6 ml of CHCl3/MeOH (1:3), and the eluates were dried under nitrogen. The samples were resuspended with 1 × 50 μl and then 1 × 100 μl of CHCl3/MeOH (1:2.2) and transferred to a 1.5-ml tube. The samples were dried down in a SpeedVac and resuspended in 20 μl of MeOH.
Quantitation of GSL Derivatives by High-Performance Liquid Chromatography (HPLC)
GSL were analyzed according to Neville et al. (2004). To dried lipid extracts, 10 μl of incubation buffer (1 mg/ml sodium cholate in 50 mM sodium acetate, pH 5.0) was added. After vigorous vortexing and spinning in a benchtop picofuge, 10 μl of 50 mU/10 μl ceramide glycanase (E.C. 3.2.1.123; Calbiochem) in incubation buffer was added to cleave the glycans. Glucosylceramide is partially digested because of the specificity of the glycanase (Wing et al., 2001). After this period, 10 μl of water was added to each enzyme digest followed by 80 μl of anthranilic acid and sodium cyanoborohydride to each digest and incubated in the 80°C oven for 1 h. Derivatized oligosaccharides were then purified on DPA-6S (Supelco, Bellefonte, PA) columns preequilibrated with 2 × 1 ml CH3CN. One milliliter of 97:3 CH3CN/H2O was added to each sample and vortexed before loading the samples onto the columns. Columns were washed with 1 ml of 99:1 CH3CN/H2O four times and then with 0.5 ml of 97:3 CH3CN/H2O, and the derivatized oligosaccharides were eluted with 0.6 ml water two times into screw-cap Eppendorfs and stored at 4°C in the dark until ready for normal phase-HPLC.
Mass Spectrometry
Matrix-assisted laser desorption ionization (MALDI) mass spectrometry was performed as described previously (Hunnam et al., 2001). Briefly, a mixture of the sample (1 μl; 100 pmol) and matrix (1 μl of a saturated solution of 2,5-dihydroxybenzoic acid in acetonitrile) was crystallized on the MALDI target. Positive ion reflectron MALDI spectra were obtained with a Micromass TOFSpec 2E mass spectrometer equipped with delayed extraction (Micromass UK, Wythenshawe, Manchester, United Kingdom). The instrument was operated as follows: accelerating voltage, 20 kV; pulse voltage, 3200 V; and laser repetition rate of 10 Hz and calibrated externally with hydrolyzed dextran sugars.
Immunofluorescence Methods
HeLa cells were fixed at room temperature for 20 min in 3% paraformaldehyde, quenched with 30 mM glycine, permeabilized with 0.1% Tx-100, incubated with the indicated primary or secondary antibodies, mounted, and viewed by confocal microscopy (Leica Microsystems, Mannheim, Germany). For Tx-100 extraction on living cells, the cells were put on ice, washed once with PBS containing 1 mM MgCl2 and 0.5 mM CaCl2, and incubated for 1 min in 1% Triton/PIPES buffer (80 mM PIPES, pH 6.8, 5 mM EGTA, and 1 mM MgCl2). The buffer was then removed and 3% paraformaldehyde was added for 15 min at room temperature as described previously (Falguieres et al., 2001).
RESULTS
The Toxicity of SLTx Is Completely Abrogated upon Treatment with NB-DGJ, an Inhibitor of Glycosphingolipid Biosynthesis
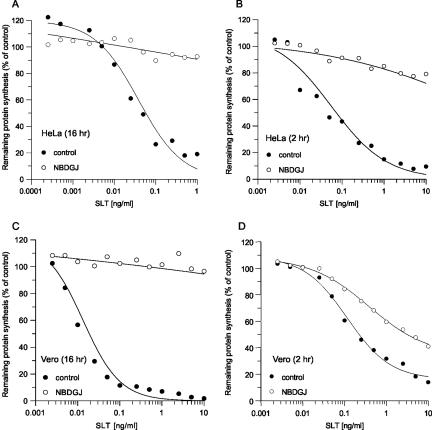
For a productive intoxication of susceptible mammalian cells, SLTx is required to bind its GSL receptor, Gb3, for uptake and delivery to the ER lumen from where the toxin is dislocated to the cytosol to inactivate ribosomes (Sandvig and van Deurs, 2002; Smith et al., 2004). Because treatment of fibroblasts and macrophages with NB-DGJ redirects endocytosed GSL (LacCer), away from the Golgi and into lysosomes (Sillence et al., 2002), we investigated whether NB-DGJ could similarly alter the cell entry and therefore the potency of SLTx. The cytotoxic effect of SLTx on cellular protein biosynthesis was measured by incubating cells with increasing concentrations of the toxin (Figure 1). When HeLa and Vero cells were pretreated with NB-DGJ for 16 h before a 4-h toxin challenge in the continued presence of the drug, cellular protein synthesis was unaffected (Figure 1, A and C). Similar protection was seen with DL-1-phenyl-2-palmitoylamino-3-morpholino-1-propanol, a chemically unrelated inhibitor of glucosylceramide synthesis (our unpublished data). A significant protection was also seen after a short 2-h pretreatment with the drug (Figure 1, B and D). Because HeLa cell sensitivity to SLTx could be modulated by NB-DGJ in a time- and concentration-dependent manner (our unpublished data), with maximal protection seen after 2 h with 100 μM NB-DGJ (Figure 1B), all subsequent experiments were performed under these conditions.
Figure 1.
NB-DGJ protects both HeLa and Vero cells from SLTx. HeLa (A and B) and Vero (C and D) cells were pretreated with either media (control, •) or 100 μM NB-DGJ (○) for either 16 h (A and C) or 2 h (B and D). After pretreatment, cells were washed and incubated with increasing concentrations of SLTx for 4 h at 37°C. Incorporation of [35S]methionine was used to determine remaining cellular protein synthesis, compared with untreated cells. The means of three independent experiments are shown. SEs were <10%.
Because NB-DGJ is an inhibitor of GSL biosynthesis, both GSL composition and binding studies were performed with SLTx after a 2-h pretreatment with 100 μM NB-DGJ. Analysis of the GSL composition in HeLa cells treated with 100 μM NB-DGJ (2 h) showed that the total levels of Gb3 remained unaltered, whereas the levels of GlcCer decreased to ∼38% of control cell levels, with reductions also seen in the levels of II3-α-N-acetylneuraminyllactosylceramide (GM3) and neolactotetraglycosylceramide (nLc4) (Table 1). Consistent with this, Scatchard analysis demonstrated that the binding affinity and number of SLTx receptor sites (i.e., Gb3) remained essentially unaltered compared with controls (Table 2). Interestingly, the levels of LacCer, the next GSL to be synthesized sequentially from GlcCer, remained unaltered after NB-DGJ treatment (Table 1). Analysis of the Tx-100-resistant fraction isolated from NB-DGJ-treated cells highlighted changes within the level of all the GSL examined, with the exception of LacCer, with significant changes occurring for GlcCer and Gb3, implying that inhibition of GSL biosynthesis rapidly altered the composition of these lipids in DRM. Cells remained sensitive to SLTx when NB-DGJ was coadministered with the toxin; furthermore, NB-DGJ pretreatment had no effect on the cytotoxicities of two other ER-directed protein toxins, Pseudomonas exotoxin A and ricin (our unpublished data). Together, these findings rule out as explanations for the SLTx-resistance phenotype a lack of surface receptors, a perturbation of the toxin uptake pathways, and, because of the identical catalytic mechanism of SLTx and ricin (Furutani et al., 1992), a direct inhibitory effect of the drug upon the SLTx active site.
Table 1.
GSL composition in HeLa cells in the presence of NB-DGJ
| Control | NB-DGJ (2 h) | NB-DGJ (16 h) | |
|---|---|---|---|
| Total levels of GSL examineda | |||
| Gb3 | 1.00 ± 0.40 | 1.00 ± 0.30 | 0.50 ± 0.10 |
| GlcCer | 2.60 ± 0.10b | 1.00 ± 0.10b | 0.60 ± 0.10 |
| LacCer | 1.00 ± 0.50c | 0.60 ± 0.20c | 0.60 ± 0.20 |
| GM3 | 1.00 ± 0.30 | 0.40 ± 0.20 | 0.40 ± 0.10 |
| nLc4 | 0.20 ± 0.06 | 0.10 ± 0.05 | 0.10 ± 0.04 |
| Sialyl- nLc4 | 0.40 ± 0.10 | 0.20 ± 0.10 | 0.10 ± 0.05 |
| Tx-100-resistant levels of GSL examineda | |||
| Gb3 | 71 ± 8d | 37 ± 9d | 34 ± 8 |
| GlcCer | 101 ± 3e | 51 ± 2e | 43 ± 1 |
| LacCer | 39 ± 2f | 27 ± 6f | 27 ± 5 |
| GM3 | 35 ± 2 | 10 ± 1 | 10 ± 1 |
| nLc4 | 13 ± 1 | 5 ± 1 | 6 ± 2 |
| Sialyl- nLc4 | 10 ± 1 | 6 ± 1 | 4 ± 1 |
Picomoles per microgram of protein (±SE); n = 3-7.
Extremely statistically significant, two-tailed p value < 0.0001.
Not statistically significant, two-tailed p value = 0.4104.
Statistically significant, two-tailed p value = 0.0179.
Extremely statistically significant, two-tailed p value < 0.0001.
Not statistically significant, two-tailed p value = 0.1066.
Table 2.
Binding of SLT-1 to HeLa cells
| Kda (nM) | Gb3 binding sites/cella | |
|---|---|---|
| Control | 81.1 ± 26.6 | 13.1 ± 6.6 × 106 |
| NB-DGJb | 62.7 ± 24.5 | 11.9 ± 5.0 × 106 |
Determined using standard Scatchard analysis, n = 3.
HeLa cells pretreated with 100 μM NBDGJ for 2 h at 37°C.
Trafficking of SLTx to the ER Is Not Perturbed by NB-DGJ Treatment
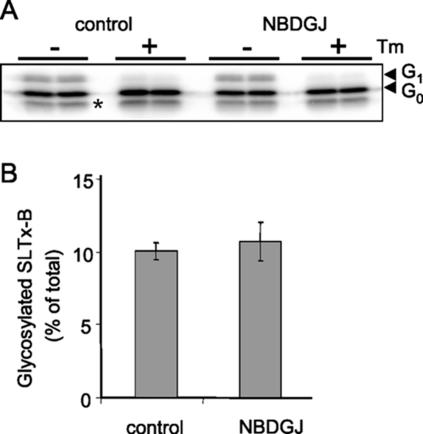
Productive intoxication with SLTx requires the initial binding of toxin to surface Gb3 molecules followed by retrograde transport of the complex to the ER. The length of the Gb3 fatty acyl chain is important for efficient ER targeting of the toxin, with the presence of the shorter chain (C16–C18) isoforms being associated with enhanced cellular sensitivity and ER targeting of SLTx (Sandvig et al., 1994; Arab and Lingwood, 1998). We therefore examined the possibility that NB-DGJ treatment was altering the availability of shorter chain Gb3 isoforms, thereby disrupting transport to the ER. However, mass spectrometric analysis of the chain lengths of Gb3 isoforms isolated after NB-DGJ treatment showed no significant difference compared with control cells (Table 3). Because treatment with NB-DGJ is known to alter trafficking of LacCer to the lysosomes in certain cell types (Sillence et al., 2002), we next examined whether the intracellular routing of SLTx had been altered after NB-DGJ treatment. The routing of toxin to the ER can be monitored and quantified using a radiolabeled SLTx-B chain variant engineered to contain a glycosylation sequence that becomes posttranslationally core glycosylated upon arrival in the ER (Johannes et al., 1997). Using such an approach, the amounts of core glycosylated, radiolabeled SLTxB were identical in NB-DGJ-treated HeLa cells and in control cells (Figure 2). The core-glycosylated SLTxB moieties disappeared upon tunicamycin treatment of the cells (Figure 2A), confirming that the proteins detected had become glycosylated, a modification that is only possible within the ER lumen. This clearly demonstrated that SLTx transport to the ER is unaltered by NB-DGJ treatment. It should be noted that this atypical, posttranslational N-glycosylation of a protein entering the ER from the Golgi is generally inefficient. The proportion of SLTx glycosylated in this way is on a par with previously reported levels (Falguieres et al., 2001).
Table 3.
Effect of NB-DGJ on the fatty acid composition of DRM and non-DRM-associated Gb3
| Control
|
NB-DGJ (2 h)
|
|||
|---|---|---|---|---|
| Gb3 species | DRMa | Soluble | DRM | Soluble |
| C24 | 42 ± 1b | 43 ± 2 | 46 ± 1 | 46 ± 1 |
| C24:1 | 8 ± 2 | 7 ± 2 | 5 ± 1 | 3 ± 2 |
| C22 | 35 ± 2 | 35 ± 1 | 36 ± 1 | 31 ± 3 |
| C18 | 4 ± 1 | 4 ± 1 | 4 ± 1 | 4 ± 1 |
| C16 | 6 ± 2 | 9 ± 3 | 7 ± 2 | 8 ± 3 |
DRM were isolated in cold Tx-100 from confluent monolayers (5 mg of protein).
Gb3 species expressed as a mean percentage of total fatty acids ± SE, n = 3-4 experiments.
Figure 2.
Transport of SLTx-B to the ER occurs in cells treated with NB-DGJ. Transport to the ER was examined by glycosylation analysis. Iodinated SLTx-B (50 nM) was internalized into control HeLa cells or NB-DGJ (100 μM; 2 h)-treated HeLa cells for 4 h. Cells were lysed, and the lysates were analyzed by gel electrophoresis (A). Tunicamycin (Tm) treatment of cells to inhibit N-glycosylation was performed before toxin internalization. G0 and G1 indicate the unmodified and the ER glycosylation product, respectively. The lower band (*) is a proteolytic cleavage product. (B) Quantitation of three independent experiments.
SLTx Can Be Associated with DRM in the ER Membrane
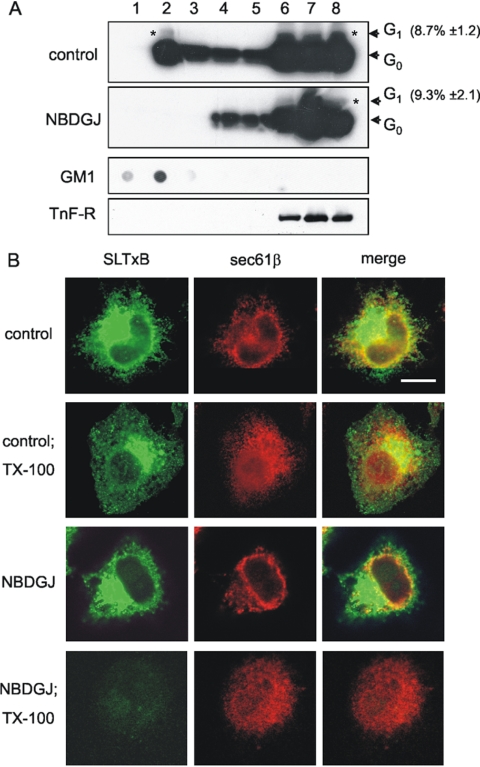
Because SLTx was still efficiently transported to the ER after NB-DGJ treatment, we hypothesized that the mechanism through which protection against the toxin was observed may reside at the level of the ER. ER targeting of SLTx has been correlated to an association with DRM at the plasma membrane (Falguieres et al., 2001; Hoey et al., 2003). This association persisted through to the ER, as shown by the presence of ER-glycosylated SLTxB-KDEL in DRM (Falguieres et al., 2001). It was therefore pertinent to test whether SLTx persisted within DRM in the ER membrane after NB-DGJ treatment. To examine this, iodinated “glycosylatable” SLTxB was bound and internalized into HeLa cells for 4 h after pretreatment with NB-DGJ. DRM association of ER-localized (i.e., glycosylated) SLTxB was investigated using the technique of buoyant density-gradient centrifugation after extraction in the presence of cold Tx-100, a widely accepted method for the isolation of DRM (Brown and London, 2000; Simons and Toomre, 2000). Gradient fractions were isolated and analyzed for the presence of the ER-localized glycosylated SLTxB. Our data confirmed the previous observation (Falguieres et al., 2001) that in control cells, a proportion of the ER-glycosylated SLTxB was localized to DRM (Figure 3A, asterisk, top). This result was supported visually. After 4 h of toxin treatment, a significant amount of fluorescently labeled SLTx could be seen within the reticular and perinuclear ER structures, along with a proportion of the ER marker, Sec61β (Figure 3B, top row). After treatment with cold 1% Tx-100, a fraction of the SLTx and Sec61β was extracted, but a significant proportion remained, and the two proteins displayed a degree of overlap around the perinuclear and peripheral ER area (Figure 3B, second row). This suggested that they had a specific distribution within the ER, possibly reflecting their presence in lipid microdomains in the ER membrane before detergent treatment. On treating cells with NB-DGJ followed by internalization of iodinated SLTxB, no glycosylated SLTxB was detectable within the DRM fraction (Figure 3A, second panel, lane 2). Nevertheless, the same proportion of SLTxB became glycosylated as in the untreated cells (Figure 3A, top). Transport to the ER after NB-DGJ treatment was also supported by the localization of SLTx within perinuclear reticular structures (Figure 3B, third row). However, after cold Tx-100 extraction, SLTx was completely solubilized from NB-DGJ-treated cells, whereas an appreciable proportion of the Sec61β remained (Figure 3B, fourth row). Together, these data revealed that a proportion of SLTx is normally found with DRM within the ER and that this association was abolished upon treatment with NB-DGJ.
Figure 3.
ER-localized glycosylated SLTx-B is present in DRM, an association that is perturbed by NB-DGJ. (A) Iodinated SLTx-B was bound and internalized into either control or NB-DGJ (100 μM; 2 h)-treated HeLa cells for 4 h. Cells were then washed and mechanically lysed, with the PNS being extracted in 1% Tx-100 buffer. DRM were floated in OptiPrep step gradients. The gradients were fractionated, and the presence of the SLTx-B was determined by gel electrophoresis. G0 and G1 (*) indicate the unmodified and the ER-located glycosylation product, respectively. Quantitation of G1 (% of total) was determined from three independent experiments. Gradients were also analyzed by dot-blot for GM1 (DRM marker) and by gel electrophoresis and blotting for a non-DRM marker, the transferrin receptor (TnF-R). (B) Cy2-coupled SLTx-B was bound to the plasma membrane of control or NB-DGJ (100 μM; 2 h)-treated HeLa cells on ice and washed and shifted to 37°C for 4 h. Cells were then washed and placed on ice, incubated (Tx-100) or not for 1 min in cold 1% Tx-100-containing buffer, and then fixed and permeabilized. Cells were then probed with a primary Sec61β antibody, washed, and incubated with an Alexa 633-coupled secondary antibody, mounted, and viewed by confocal microscopy. Bar, 5 μm.
NB-DGJ Alters the Recruitment or Association of SLTx into DRM
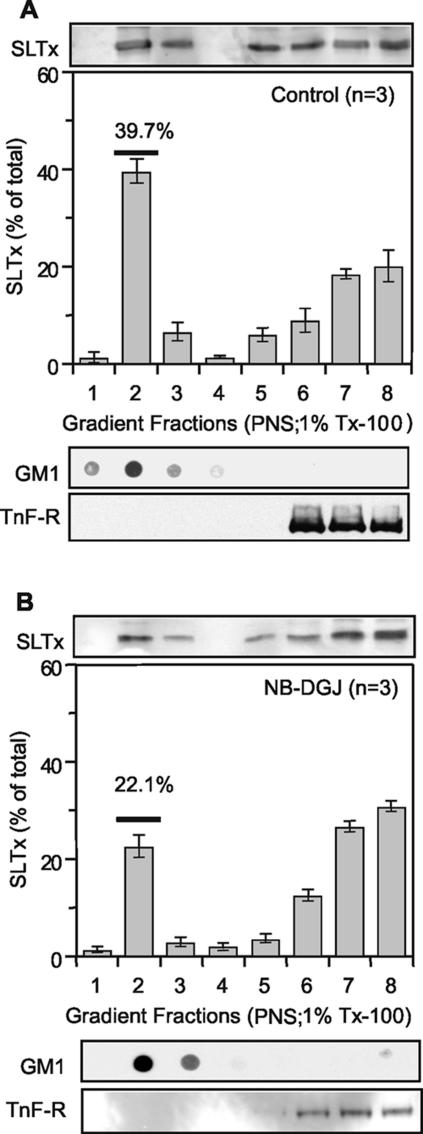
Because NB-DGJ treatment abolished DRM association of SLTx within the ER membrane, we next investigated DRM association of SLTx bound at the cell surface at 4°C. In agreement with a previous study (Falguieres et al., 2001), ∼40% of the bound SLTx was sequestered within the isolated GM1-containing DRM fraction of untreated cells as determined using resistance to extraction in cold 1% Tx-100 and subsequent flotation based on buoyant density (Figure 4A). Interestingly, the level of SLTx found with the DRM fraction was reduced from 40 to 22% after treatment of cells with the drug (Figure 4B). This correlated well with the halving of DRM-sequestered Gb3 after NB-DGJ treatment (Table 1). However, although there was less toxin associated with isolated DRM, enough SLTx remained within such lipid microdomains in the plasma membrane in vivo to permit transport to the ER. Interestingly, the DRM association of SLTxB observed in macrophages, where toxin is routed to the lysosome, was <2% of the total toxin bound (Falguieres et al., 2001).
Figure 4.
NB-DGJ reduces the association of SLTx with cell surface DRM. SLTx (1 μM) was bound to control HeLa cells (A) or NB-DGJ treated (2 h; 100 μM) HeLa cells (B) on ice. The cells were washed and mechanically lysed with the resulting PNS incubated with 1% Tx-100. DRM were floated in OptiPrep step gradients. The gradients were fractionated and the presence of bound SLTx was determined by gel electrophoresis and Western blotting and quantified using enhanced chemifluorescence. Gradients were also analyzed by dot-blot for GM1 (DRM marker) and by gel electrophoresis and blotting for a non-DRM marker, the transferrin receptor (TnF-R). Means of three experiments (±SEM) are shown.
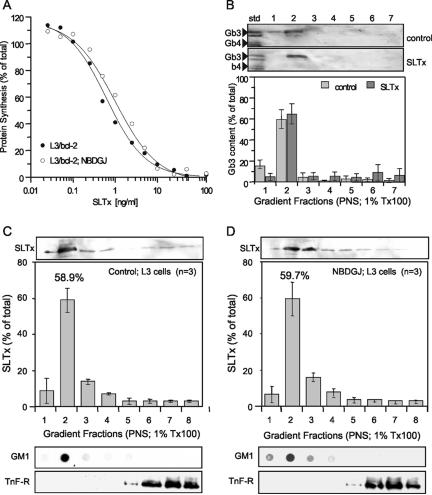
If the recruitment of Gb3 into lipid rafts occurs upon toxin binding in HeLa cells, as has been suggested (Falguières and Johannes, unpublished data), then a prediction arising from this is that NB-DGJ treatment should have little or no effect on Gb3 molecules already associated with lipid rafts before toxin binding and therefore have correspondingly little or no effect on SLTx cytotoxicity. Indeed, the cytotoxicity observed with SLTx was not significantly altered after either a 2-h (Figure 5A) or 16-h (our unpublished data) NB-DGJ pretreatment of a Burkitt's lymphoma derived-line, L3/bcl-2, in which a high level of Gb3 is found associated at all times within the isolated DRM fraction (Figure 5B). This implied that the binding of ligand to Gb3 did not alter its DRM association in this cell type. The amount of Gb3 found with DRM was not affected after drug treatment (our unpublished data). Indeed, analysis of L3/bcl-2 cells shows that after a 2-h treatment with NB-DGJ, there was no significant effect on the levels of the GSL examined (Table 4). However, after 16 h the level of GlcCer was decreased by ∼50% showing that L3/bcl-2 cells were nevertheless sensitive to the drug. Furthermore, there was no change in the level of SLTx found in the DRM fraction after NB-DGJ treatment compared with control cells (Figure 5, C and D). This was in marked contrast to the situation seen in HeLa cells (Figure 4). These findings support our prediction that NB-DGJ perturbs the recruitment to and/or the affinity of SLTx/Gb3 complexes to associate with lipid rafts at the cell surface.
Figure 5.
NB-DGJ does not affect the cytotoxicity of SLTx in L3/bcl-2 cells that maintain Gb3 receptors in DRM. (A) L3/bcl-2 cells were pretreated with either media (•) or 100 μM NB-DGJ (○) for 2 h before incubation with increasing concentrations of SLTx for 4 h at 37°C. Incorporation of [35S]methionine was used to determine remaining cellular protein synthesis, compared with untreated cells. SEs were <10%. (B) Analysis of Gb3 association with DRM in L3/bcl-2 cells on binding of SLTx. Ice-cold L3/bcl-2 control cells or SLTx (1 μM)-bound cells were washed, mechanically lysed, DRM extracted in 1% Tx-100 buffer, and floated in OptiPrep step gradients. The gradients were fractionated, and the neutral GSLs were extracted and separated by TLC. The presence of Gb3 in the fractions was detected (top) and quantified (graph) by overlay with SLTxB and ECF. (C) SLTx (1 μM) was bound to control L3/bcl-2 cells or (D) to NB-DGJ-treated (2 h; 100 μM) L3/bcl-2 cells on ice. The cells were washed and mechanically lysed with the resulting PNS incubated with 1% Tx-100. DRM were floated in OptiPrep step gradients. The gradients were fractionated, and the presence of bound SLTx was determined by gel electrophoresis and Western blotting and quantified using enhanced chemifluorescence. Gradients were also analyzed by dot-blot for GM1 (DRM marker) and by gel electrophoresis and blotting for a nonraft marker, the transferrin receptor (TnF-R). Means of three experiments (±SEM) are shown.
Table 4.
GSL composition in L3/bcl-2 cells in the presence of NB-DGJ
| Total levels of GSL examineda
|
||||
|---|---|---|---|---|
| Control | NB-DGJ (2 h) | NB-DGJ (16 h) | CsA (2 h) | |
| Gb3 | 14.0 ± 1.0 | 17.0 ± 2.0 | 13 ± 1 | 19 ± 2 |
| GlcCer | 15.0 ± 4.0 | 19.0 ± 4.0 | 8 ± 2 | 17 ± 5 |
| LacCer | 3.0 ± 1.0 | 3.0 ± 1.0 | 4 ± 1 | 3 ± 1 |
| GM3 | 13.0 ± 1.0 | 14.0 ± 1.0 | 18 ± 1 | 14 ± 1 |
| Gb4 | 0.2 ± 0.1 | 0.2 ± 0.1 | 0.3 ± 0.1 | 0.2 ± 0.1 |
Picomoles per microgram of protein (±SE); n = 3.
DRM Association of SLTx/Gb3 Can Be Influenced by the Levels of Glucosylceramide
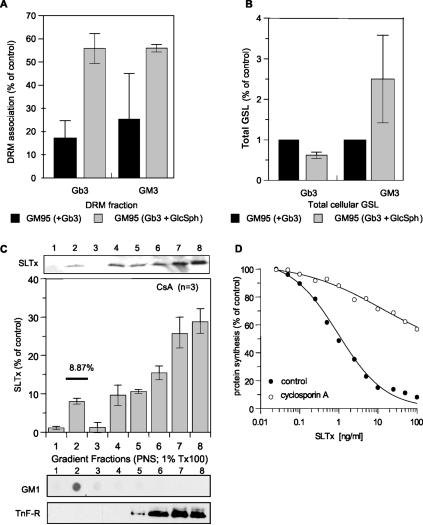
Because NB-DGJ specifically inhibits ceramide glucosyltransferase (Platt et al., 1994), an enzyme that catalyzes the first step in GSL biosynthesis located in the cis-Golgi, it was not immediately obvious how this drug was able to perturb recruitment of SLTx into lipid rafts at the plasma membrane. However, this phenomenon might be explained by the levels of available GlcCer in the cell. NB-DGJ inhibits synthesis of GlcCer from ceramide, thus reducing production of GSL (Platt et al., 1994; Andersson et al., 2000). Indeed, after treatment with NB-DGJ the DRM-associated GlcCer was reduced by ∼50% in a 2-h period in HeLa cells (Table 1), whereas the level of LacCer in DRM—the GSL directly synthesized from GlcCer—was not significantly changed (Table 1). We reasoned that the level of GlcCer may therefore influence the affinity of surface-bound SLTx for DRM. To address this, we introduced exogenous Gb3 into GM95 cells that have very low levels of endogenous GSL and that completely lack Gb3 (Ichikawa et al., 1994). After overnight incubation, ∼20% of the Gb3 became sequestered into the DRM fraction after toxin binding (Figure 6A). When GM95 cells were coincubated with Gb3 and glucosylsphingosine (GlcSph), a compound that can be acylated to increase the concentration of cellular GlcCer (Farrer and Dawson, 1990), the amount of toxin-bound Gb3 in DRM increased to >50% (Figure 6A). The increased population of the complex within DRM did not arise from a conversion of GlcCer to Gb3, because GM95 cells lack Gb3 synthase (Ichikawa et al., 1994). In accordance with this, an increase in the total level of Gb3 was not observed (Figure 6B). By contrast, cellular II3-α-N-acetylneuraminyllactosylceramide (GM3) levels did increase after GlcSph treatment (Figure 6B), showing that the additional GlcCer generated from the added GlcSph had allowed an increase in the levels of other GSL. These data suggest that the levels of GlcCer can influence, directly or indirectly, the amount of bound toxin that becomes recruited to DRM.
Figure 6.
The association of SLTx/Gb3 complexes with DRM is influenced by other GSL. GM95 cells were incubated with 20 μM Gb3 for 48 h in the presence or absence of 20 μM GlcSph. The cells were washed, lysed in 0.5% Tx-100 buffer, and DRM were floated on step gradients. The gradients were fractionated, and the levels of Gb3 and GM3 in both the DRM fraction (A) and the total cellular fraction (B) were quantified. (C) Cyclosporin A-treated (2 h; 10 μM) HeLa cells were incubated with 1 μM SLTx for 30 min on ice. The cells were washed, mechanically lysed, DRM extracted in 1% Tx-100 buffer, and floated in OptiPrep step gradients. The gradients were fractionated, and the presence of bound SLTx was determined by gel electrophoresis and Western blotting and quantified using enhanced chemifluorescence. Gradients were also analyzed by dot-blot for GM1 (DRM marker) and by gel electrophoresis and blotting for a non-DRM marker, the transferrin receptor (TnF-R). Means of three experiments (±SEM) are shown. (D) HeLa cells were pretreated with either media (•) or 10 μM cyclosporin A (○) for 2 h before incubation with increasing concentrations of SLTx for 4 h at 37°C. Incorporation of [35S]methionine was used to determine remaining cellular protein synthesis, compared with untreated cells. SEs were <10%.
We then modulated the activity of P-glycoprotein (MDR-1) because it is known that overexpression of MDR-1 leads to an increased translocation of GlcCer into the Golgi lumen (Lala et al., 2000; Eckford and Sharom, 2005), raising the supply of this lipid for the synthesis of other GSL, including Gb3. We reasoned that the effect of inhibiting MDR-1 activity should therefore be similar to that seen for NB-DGJ treatment. Indeed, when we examined the amount of SLTx associated with DRM after a 2-h pretreatment of HeLa cells with the well characterized MDR-1 inhibitor cyclosporine A (CsA) (Lavie et al., 1997; Landwojtowicz et al., 2002; Eckford and Sharom, 2005), it was found to be reduced (Figure 6C) compared with control cells (Figure 4A). Furthermore, when HeLa cells were pretreated with CsA (10 μM; 2 h), they displayed appreciable resistance to a toxic challenge with SLTx (Figure 6D). GSL analysis revealed that treatment of HeLa cells with CsA (10 μM; 2 h) reduced both the total levels and DRM association of GlcCer (Table 5). DRM-associated Gb3 was also reduced after CsA treatment, but the total cellular pool remained unaffected (Table 5). Interestingly, no protection from SLTx cytotoxicity was observed in L3/bcl-2 cells treated with CsA (10 μM; 2 h) (our unpublished data), a finding that correlated well with the unperturbed levels of GlcCer and Gb3 in these cells (Table 4). Together, the data suggest that the degree of DRM association of SLTx/Gb3 can be influenced by the levels of GlcCer.
Table 5.
GSL composition in HeLa cells in the presence of the MDR-1 inhibitor cyclosporin A
| Control | CsA (2h) | |
|---|---|---|
| Total levels of GSL examineda | ||
| Gb3 | 1.0 ± 0.4 | 1.0 ± 0.2 |
| GlcCer | 2.6 ± 0.1b | 0.6 ± 0.2b |
| LacCer | 1.0 ± 0.5c | 0.40 ± 0.1c |
| GM3 | 1.0 ± 0.3 | 0.40 ± 0.1 |
| nLc4 | 0.20 ± 0.06 | 0.20 ± 0.06 |
| Sialyl-nLc4 | 0.4 ± 0.1 | 0.60 ± 0.20 |
| Tx-100 resistant levels of GSL examined1 | ||
| Gb3 | 71 ± 8d | 17 ± 1d |
| GlcCer | 101 ± 3e | 19 ± 4e |
| LacCer | 39 ± 2 | 6 ± 1 |
| GM3 | 35 ± 2 | 3 ± 1 |
| nLc4 | 13 ± 1 | 4 ± 1 |
| Sialyl-nLc4 | 10 ± 1 | 13 ± 2 |
Picomoles per microgram of protein (±SE); n = 3-7.
Extremely statistically significant, two-tailed p value = 0.0002.
Not statistically significant, two-tailed p value = 0.2288.
Extremely statistically significant, two-tailed p value = 0.0005.
Extremely statistically significant, two-tailed p value < 0.0001.
SLTx Requires ER-DRM Association for Subsequent Intoxication
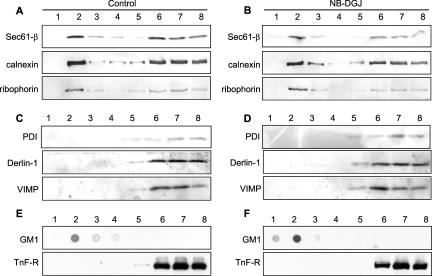
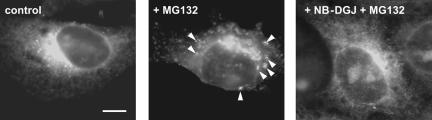
Because the effect of NB-DGJ is to significantly mitigate toxin potency, we propose that SLTx must require an interaction with DRM in the ER membrane for some crucial step that is obligatory for toxin entry to the cytosol. Because SLTx is thought to co-opt proteins involved in the retro-translocation process associated with ER-associated degradation (ERAD) (Ellgaard and Helenius, 2003; Helenius and Aebi, 2004), we isolated and analyzed DRM fractions for the presence of these proteins. A proportion of calnexin, ribophorin-1, and Sec61β showed coincidence with the DRM fraction in both control (Figure 7A) and NB-DGJ-treated HeLa cells (Figure 7B), whereas this was not seen for PDI, Derlin-1, or VIMP in either control (Figure 7C) or in NB-DGJ-treated cells (Figure 7D). The fraction of Sec61β in DRM (Figure 7) was similar to that previously visualized after detergent extraction (Figure 3B). Recently, a subcompartment of the ER has been identified in which a membrane-bound ERAD substrate, the precursor of human asialoglycoprotein receptor H2a, accumulated to be observed as punctate structures within the ER upon inhibition of proteasomes in the cytosol (Kamhi-Nesher et al., 2001). We therefore examined the distribution of Cy2-labeled SLTx internalized for 4 h into HeLa cells after a 5-h inhibition of proteasomes using 10 μM MG132 (Figure 8). SLTx staining revealed a general reticular pattern consistent with an ER localization in untreated cells (Figure 8, left), whereas toxin was observed to accumulate in punctate structures, restricted to juxtanuclear and peripheral regions of ER in MG132-treated cells (Figure 8, middle). This accumulation was consistent with that seen for H2a under identical conditions of proteosomal inhibition (Kamhi-Nesher et al., 2001). This punctate staining of SLTx was lost after proteasome inhibition and NB-DGJ treatment. Instead, a more general ER reticular staining was revealed (Figure 8, right). These data suggest that NB-DGJ treatment that reduces the cellular level of GlcCer perturbs the ability of SLTx to localize in areas of the ER that are compatible for subsequent retro-translocation to the cytosol.
Figure 7.
A proportion of some ER-resident proteins involved with ERAD and retro-translocation are found in DRM. Untreated HeLa cells (A and C) or NB-DGJ-treated (2 h; 100 μM) HeLa cells (B and D) were washed, lysed in 1% Tx-100 buffer, and DRM were floated in OptiPrep step gradients. The gradients were fractionated, and the location of the various ER-resident proteins was determined by gel electrophoresis and Western blotting with specific antisera. Gradients were also analyzed by dot-blot for GM1 (DRM marker) and by gel electrophoresis and blotting for a non-DRM marker, the transferrin receptor (TnF-R). (E) Untreated. (F) NB-DGJ-treated cells.
Figure 8.
ER-localized SLTx accumulates at discrete locations upon inhibition of proteasomes. Cy2-coupled SLTx was bound to the plasma membrane of control (left) or MG132 (10 μM; 5 h)-treated (middle), or MG132- (10 μM; 5 h) and NB-DGJ (100 μM; 2 h)-treated (right) HeLa cells on ice. The cells were washed and shifted to 37°C for 4 h. Cells were washed and placed on ice and then fixed and permeabilized. Cells were washed and mounted and viewed by confocal microscopy. Arrowheads indicate the accumulation in punctate regions of the ER. Bar, 5 μm.
DISCUSSION
In this study, we have shown that specific inhibition of ceramide glucosyltransferase with NB-DGJ (Platt et al., 1994) completely protected both HeLa and Vero cells from subsequent intoxication by SLTx. Under the conditions used here, treatment with NB-DGJ altered neither the binding affinity of the toxin to its GSL receptor Gb3 nor the overall number of receptors. Treatment did lower the recruitment of SLTx into detergent resistant membranes at the cell surface, although presumably, sufficient association remained to permit retrograde transport to the ER (Falguieres et al., 2001; Nichols et al., 2001; Rodighiero et al., 2001; Fujinaga et al., 2003; Hoey et al., 2003; Lencer and Tsai, 2003). However, NB-DGJ abolished DRM association of SLTx within the ER. Because we interpret DRM to represent lipid microdomains in the original membrane, we propose that the absence of SLTx from such microdomains in the ER membrane is the most likely explanation for the acquired resistance of NB-DGJ-treated cells to this lipid-binding toxin.
A number of groups have now provided good evidence for the existence of lipid rafts or microdomains in cell membranes (Friedrichson and Kurzchalia, 1998; Harder et al., 1998; Varma and Mayor, 1998; Pralle et al., 2000; Wilson et al., 2000). The inclusion or exclusion of certain lipids and proteins and the affinity of a given protein for such rafts can result in a locally different character of the lipid microdomain compared with the surrounding membrane, giving rise to a variety of different cellular functions (London and Brown, 2000). Although lipid microdomains on the cell surface tend to be transient (Schutz et al., 2000), relatively stable microdomains have been visualized in the endocytic pathway (Sharma et al., 2003) where they are proposed to play a role in protein and lipid sorting (Gruenberg, 2001). Although it is not possible to isolate lipid microdomains or rafts in their native state, the dense packaging of GSL to form liquid ordered (lo) phases in artificial membranes, and by extension lo domains in cell membranes, gives rise to a characteristic detergent resistance. This in turn allows for a simple biochemical purification of DRM. These are generally interpreted as representing lipid microdomains/rafts and as such they have been used to indicate whether a particular protein associates with lipid rafts in vivo (London and Brown, 2000). However, the physical basis for the extraction of lipids with detergents is poorly understood, and care should be taken in experimental interpretation and any extrapolation to their in vivo function. Furthermore, it is unlikely that DRM isolated from cells accurately reflect preexisting structures or organization of the membrane. That said, however, the ability to partition with the DRM fraction could reflect an important membrane-related biochemical property of the specific component in question.
We have shown that recruitment and partitioning of the Gb3/SLTx complex to such DRM is influenced by the availability of GlcCer. Whereas the amount of DRM-associated SLTx was reduced upon treatment of cells with either NB-DGJ or cyclosporine A (an inhibitor of the GSL flippase, MDR-1; De Rosa et al., 2004), it was increased by the addition of exogenous glycosphingosine, a precursor of GlcCer. Indeed, previous transfection of Madin-Darby canine kidney (MDCK) cells with the gene for MDR-1, whose protein product would increase the availability of GlcCer, dramatically increased the sensitivity of these cells to the action of SLTx (Lala et al., 2000). It has been reported that some types of GSL are capable of modulating the association of specific lipids and proteins within DRM. GlcCer depletion of RAW macrophages increased the detergent resistance of the specific GSL, GD1a (Sillence et al., 2002). The association of Src proteins with DRM in Lewis lung carcinoma cells was eliminated after depletion of GlcCer, LacCer, and GM3 (Inokuchi et al., 2000). An in vitro study has also shown that in artificial liposomes containing LacCer, GPI-anchored membrane dipeptidase was sequestered into DRM. However, liposomes alone, or liposomes enriched with different GSL, failed to support DRM association of this protein (Parkin et al., 2001). This type of modulation may also occur in the opposite direction. For example, addition of GM1 to MDCK cells rendered a GPI-anchored form of the growth hormone decay accelerating factor protein that was originally detergent resistant, now susceptible to detergent extraction (Simons et al., 1999). It has been shown that preferential depletion of GSL in DRM fractions does not affect the levels of sphingomyelin or cholesterol and does not induce significant changes in the distribution of proteins (Shu et al., 2000). Therefore, the role of GSL in affecting DRM association of other constituents seems to be highly specific. How specific GSL influence whether a particular lipid or protein is found within a lipid microdomain remains unclear. It is interesting to note that an ER monohexosylceramide flippase has been identified (Buton et al., 2002). This may be effected either directly or indirectly because GlcCer transferase inhibitors can have more than one site of action (Kok et al., 1998).
Interestingly, the fatty acyl chain length of Gb3 can affect the intracellular trafficking of STx/SLTx, with binding to the shorter C16–C18 isoforms promoting transport to the ER and enhancing cytotoxicity (Sandvig et al., 1994; Arab and Lingwood, 1998). However, tight packing of GSL with long, rather than short, acyl chains is a key feature of lipid microdomain organization (Brown and London 2000). It is therefore reasonable to propose that the presence of GlcCer can increase the affinity of the shorter Gb3 isoforms that correlate with efficient ER routing and cytotoxicity of SLTx, for DRM association. The data presented here are in agreement with this hypothesis because the effects of inhibiting GSL synthesis with NB-DGJ resulted in a reduction in the amount of Gb3-bound SLTx recruited into DRM that correlated with a lower level of GlcCer. It has been demonstrated that association of raft components can be stabilized by cross-linking (Harder et al., 1998). The receptor-binding SLTx B chains are pentameric, and it is known from the crystal structure that cross-linking of Gb3 molecules occurs when this toxin binds to the lipid (Ling et al., 1998). However, our data suggest that Gb3 cross-linking alone is not sufficient to promote the association of SLTx into triton-extractable DRM, because the binding affinity of the toxin for its receptor was unaltered after NB-DGJ treatment, even though the amount found in DRM was reduced.
NB-DGJ treatment only lowered the DRM association of SLTx at the plasma membrane and had no effect on retrograde transport to the ER. However, NB-DGJ abolished toxin sequestration into DRM at the level of the ER and completely protected cells from SLTx. This suggests that DRM association of SLTx in the ER is an essential requirement for the subsequent intoxication of HeLa cells by SLTx. The gradient in the observed amount of SLTx found with DRM after drug treatment is consistent with the cis-Golgi localization of ceramide glucosyltransferase. After a 2-h inhibition of GlcCer synthesis with NB-DGJ it might be expected that levels of GlcCer in the Golgi and ER be depleted more quickly than at the plasma membrane.
To inactivate ribosomes, the catalytic A1 domain of SLTx must first retrotranslocate across the ER membrane. Little is known about the mechanistic aspects of this process, although certain interactions have been demonstrated for the related toxin CTx and for ricin. Within the ER, the catalytic A-chains of both these toxins are liberated from their respective B-subunits by the action of PDI (Tsai et al., 2001; Spooner et al., 2004). CTx-A is also unfolded by PDI before its retro-translocation (Tsai et al., 2001; Tsai and Rapoport, 2002). The unfolding of other toxins that reach the ER has not been established. However, ricin A chain (RTA) and STx-A have both been shown to interact with negatively charged phospholipid vesicles (Suhan and Hovde, 1998; Day et al., 2002). Indeed, RTA undergoes significant structural change upon interacting with such lipids (Day et al., 2002), whereas mutations in a proposed membrane-insertion domain of STx-A reduced its interaction with lipids with a concomitant effect on cytotoxicity (Suhan and Hovde, 1998; LaPointe et al., 2005). Whether these events occur within the ER is unknown, but it remains an appealing possibility that interactions with lipids play an important role in preparing some toxins for the ER membrane translocation step. The negatively charged phospholipid phosphatidylserine (PS) is present within both leaflets of the ER membrane of mammalian cells (Chang et al., 2004) and indeed can be highly enriched within DRM (Pike et al., 2002). Furthermore, it has been demonstrated that the presence of GlcCer dramatically enhanced the interaction of activated protein C with negatively charged PS vesicles (Yegneswaran et al., 2003), leading to the hypothesis that microdomains enriched in neutral GSL can promote specific phospholipid–protein interactions. Our data are clearly consistent with this. After NB-DGJ treatment, when the overall level of GlcCer is reduced, SLTx in the ER is absent from isolated DRM. As such, after subunit reduction in the ER lumen, we propose that the SLTx A-chain may not be correctly positioned to interact with negatively charged lipids either because of its nonraft location and/or the absence of GlcCer.
It is widely held that toxin retro-translocation to the cytosol is mediated by the machinery used to discharge newly made but misfolded or orphan proteins to the cytosol for destruction by proteasomes (Simpson et al., 1999; Wesche et al., 1999; Teter et al., 2002, 2003), in the process of ERAD. Lipid-induced changes in the secondary and tertiary structure of RTA have been demonstrated previously (Day et al., 2002), leading to the proposition that this might allow interception by ERAD proteins to trigger toxin retro-translocation. A similar situation may apply to SLTx/STx, although this is currently unclear. A novel quality control subcompartment of the ER for membrane-bound ERAD substrates has been identified in mammalian cells (Kamhi-Nesher et al., 2001). On perturbation of the cytosolic degradation machinery, an H2a ERAD substrate accumulated in cells alongside the ER chaperones calreticulin and calnexin (but not PDI), and was visualized by a punctate juxtanuclear staining of the ER. Pulse–chase analysis and coimmunoprecipition after the synthesis of the mutant H2a revealed an increased interaction between mutant H2a and Sec61-β, whereas total H2a levels decreased because of proteosomal degradation. It was therefore suggested that interaction with Sec61 could be the rate-limiting step during retro-translocation of ERAD substrates (Kamhi-Nesher et al., 2001). Our data show that SLTx internalized into HeLa cells gives a similar punctate juxtanuclear ER staining upon inhibition of proteasomes but not when GlcCer is depleted. Furthermore, we found ribophorin, calnexin and Sec61-β but not PDI, Derlin-1 or VIMP to be DRM-associated in HeLa cells. Recently, it has been shown that SLTx can interact with a range of ER proteins, including BiP, calnexin, and Sec61, required for transport from the ER lumen to the cytosol (Yu and Haslam, 2005).
Although detergent extraction has been widely used to postulate in vivo lipid microdomain association, a degree of caution must be taken when relating this to lipid rafts within the ER. However, the use of such methodology to study ER-resident proteins within DRM has been demonstrated previously (Sevlever et al., 1999; Falguieres et al., 2001; Li et al., 2003; Falguieres and Johannes, 2006). Indeed, it has been proposed that because of the lower content of cholesterol and GSL in the ER membrane, weaker interactions may result between ER–DRM components, such that extraction with 1% Tx-100 may actually result in an underestimation of the association of some ER components with DRM (Sevlever et al., 1999). We therefore feel that our data showing DRM association of certain ER-resident proteins, with the exclusion of others, is not artificial and could correlate with a proportion of these being partly present within liquid-or-dered microdomains in the ER membrane. Indeed, the ER-resident proteins calnexin (Sevlever et al., 1999; Li et al., 2003) and BiP (Falguieres et al., 2001; Falguieres and Johannes, 2006) have previously been isolated in DRM. However, our finding of Sec61-β within a DRM fraction contradicts a previous report of a study in yeast, where the homologue Sec61p was soluble in cold Tx-100 (Bagnat et al., 2000). This might reflect differences between the sterol-mediated ER microdomain organization of yeast and mammalian systems (Arora et al., 2004) and/or their ERAD mechanisms, such as the apparent lack of a calnexin–calreticulin cycle in yeast (Mancini et al., 2003; Helenius and Aebi, 2004). Other membrane complexes, Derlin-1 and VIMP, have recently been identified as being important for the retro-translocation of major histocompatability complex (MHC) class I molecules mediated by the human cytomegalovirus-encoded glycoprotein US11 (Lilley and Ploegh, 2004; Ye et al., 2004). Here, we show these proteins cannot be extracted within DRM. As yet, no role for the Derlin-1-containing complex in the dislocation of ER-directed toxins has been established. Furthermore, this complex seems substrate-specific because the dislocation of MHC class I molecules catalyzed by the related human cytomegalovirus-encoded glycoprotein US2 is independent of Derlin-1 (Lilley and Ploegh, 2004).
In conclusion, our study has revealed the importance of GlcCer levels in determining the fraction of SLTx/Gb3 complexes found within DRM. This interaction with Gb3 receptors in DRM seems critical not only for transport to the ER but also for a later step at the level of the ER. It is possible that the association with DRM may influence toxin retrotranslocation to the cytosol. Further work is now required to identify the precise molecular composition of ER membrane microdomains and the role(s) these play in facilitating transport of this toxin to the cytosol.
Acknowledgments
D.C.S. is funded by Wellcome Trust Programme Grant 063058/Z/00/Z to L.M.R. and J.M.L.; D.J.S. and F.M.P. are funded by the Ara Parseghian Medical Research Foundation and Action Medical Research; and R.M.J. is funded by a Biotechnology and Biological Sciences Research Council (UK) Ph.D. studentship. This work was supported by grants from the Wellcome Trust (to L.M.R. and J.M.L.), the Ligue Nationale contre le Cancer, Association de Recherche Contre le Cancer (no. 5177 and no. 3105), Fondation de France, Action Concertée Incitative-Jeunes chercheurs (no. 5233) to L. J., and fellow-ships from Ligue Nationale contre le Cancer and Fondation pour la Recherche Médicale to T. F. We thank Katherine Moore for technical help.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–11–1035) on December 28, 2005.
Abbreviations used: CTx, cholera toxin; CsA, cyclosporine A; DRM, detergent-resistant membrane(s); ER, endoplasmic reticulum; Gb3, globotriaosylceramide; GlcCer, glucosylceramide; GlcSph, glucosylsphingosine; GSL, glycosphingolipids; LacCer, lactosylceramide; NB-DGJ, N-butyldeoxygalactonojirimycin; STx, Shiga toxin; SLTx, Shiga-like toxin; SLTxB, Shiga-like toxin B-subunit; GM3, II3-α-N-acetylneuraminyllactosylceramide, nLc4, neolactotetraglycosylceramide; Sialyl-nLc4, IV3-α-N-acetylneuraminylneolactotetraglycosylceramide.
References
- Andersson, U., Butters, T. D., Dwek, R. A., and Platt, F. M. (2000). N-Butyldeoxygalactonojirimycin: a more selective inhibitor of glycosphingolipid biosynthesis than N-butyldeoxynojirimycin, in vitro and in vivo. Biochem. Pharmacol. 59, 821–829. [DOI] [PubMed] [Google Scholar]
- Arab, S., and Lingwood, C. A. (1998). Intracellular targeting of the endoplasmic reticulum/nuclear envelope by retrograde transport may determine cell hypersensitivity to verotoxin via globotriaosyl ceramide fatty acid isoform traffic. J. Cell Physiol. 177, 646–660. [DOI] [PubMed] [Google Scholar]
- Arora, A., Raghuraman, H., and Chattopadhyay, A. (2004). Influence of cholesterol and ergosterol on membrane dynamics: a fluorescence approach. Biochem. Biophys. Res. Commun. 318, 920–926. [DOI] [PubMed] [Google Scholar]
- Bagnat, M., Keranen, S., Shevchenko, A., and Simons, K. (2000). Lipid rafts function in biosynthetic delivery of proteins to the cell surface in yeast. Proc. Natl. Acad. Sci. USA. 97, 3254–3259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benting, J. H., Rietveld, A. G., and Simons, K. (1999). N-Glycans mediate the apical sorting of a GPI-anchored, raft-associated protein in Madin-Darby canine kidney cells. J. Cell Biol. 146, 313–320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bligh, E. G., and Dyer, W. J. (1959). A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 37, 911–917. [DOI] [PubMed] [Google Scholar]
- Brown, D. A., and London, E. (2000). Structure and function of sphingolipid- and cholesterol-rich membrane rafts. J. Biol. Chem. 275, 17221–17224. [DOI] [PubMed] [Google Scholar]
- Buton, X., Herve, P., Kubelt, J., Tannert, A., Burger, K. N., Fellmann, P., Muller, P., Herrmann, A., Seigneuret, M., and Devaux, P. F. (2002). Transbilayer movement of monohexosylsphingolipids in endoplasmic reticulum and Golgi membranes. Biochemistry 41, 13106–13115. [DOI] [PubMed] [Google Scholar]
- Chang, Q. L., Gummadi, S. N., and Menon, A. K. (2004). Chemical modification identifies two populations of glycerophospholipid flippase in rat liver ER. Biochemistry 43, 10710–10718. [DOI] [PubMed] [Google Scholar]
- Day, P. J., Pinheiro, T. J., Roberts, L. M., and Lord, J. M. (2002). Binding of ricin A-chain to negatively charged phospholipid vesicles leads to protein structural changes and destabilizes the lipid bilayer. Biochemistry 41, 2836–2843. [DOI] [PubMed] [Google Scholar]
- De Rosa, M. F., Sillence, D., Ackerley, C., and Lingwood, C. (2004). Role of multiple drug resistance protein 1 in neutral but not acidic glycosphingolipid biosynthesis. J. Biol. Chem. 279, 7867–7876. [DOI] [PubMed] [Google Scholar]
- Deeks, E. D., Cook, J. P., Day, P. J., Smith, D. C., Roberts, L. M., and Lord, J. M. (2002). The low lysine content of ricin A chain reduces the risk of proteolytic degradation after translocation from the endoplasmic reticulum to the cytosol. Biochemistry 41, 3405–3413. [DOI] [PubMed] [Google Scholar]
- Eckford, P. D., and Sharom, F. J. (2005). The reconstituted P-glycoprotein multidrug transporter is a flippase for glucosylceramide and other simple glycosphingolipids. Biochem. J. 389, 517–526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellgaard, L., and Helenius, A. (2003). Quality control in the endoplasmic reticulum. Nat. Rev. Mol. Cell Biol. 4, 181–191. [DOI] [PubMed] [Google Scholar]
- Endo, Y., Tsurugi, K., Yutsudo, T., Takeda, Y., Ogasawara, T., and Igarashi, K. (1988). Site of action of a Vero toxin (VT2) from Escherichia coli O 157, H7 and of Shiga toxin on eukaryotic ribosomes. RNA N-glycosidase activity of the toxins. Eur. J. Biochem. 171, 45–50. [DOI] [PubMed] [Google Scholar]
- Falguieres, T., and Johannes, L. (2006). Shiga toxin B-subunit binds to the chaperone BiP and the nucleolar protein B23. Biol. Cell 98, 125–134. [DOI] [PubMed] [Google Scholar]
- Falguieres, T., Mallard, F., Baron, C., Hanau, D., Lingwood, C., Goud, B., Salamero, J., and Johannes, L. (2001). Targeting of Shiga toxin B-subunit to retrograde transport route in association with detergent-resistant membranes. Mol. Biol. Cell 12, 2453–2468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farrer, R. G., and Dawson, G. (1990). Acylation of exogenous glycosylsphingosines by intact neuroblastoma (NCB-20) cells. J. Biol. Chem. 265, 22217–22222. [PubMed] [Google Scholar]
- Friedrichson, T., and Kurzchalia, T. V. (1998). Microdomains of GPI-anchored proteins in living cells revealed by crosslinking. Nature 394, 802–805. [DOI] [PubMed] [Google Scholar]
- Fujinaga, Y., Wolf, A. A., Rodighiero, C., Wheeler, H., Tsai, B., Allen, L., Jobling, M. G., Rapoport, T., Holmes, R. K., and Lencer, W. I. (2003). Gangliosides that associate with lipid rafts mediate transport of cholera and related toxins from the plasma membrane to endoplasmic reticulum. Mol. Biol. Cell 14, 4783–4793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furutani, M., Kashiwagi, K., Ito, K., Endo, Y., and Igarashi, K. (1992). Comparison of the modes of action of a Vero toxin (a Shiga-like toxin) from Escherichia coli, of ricin, and of alpha-sarcin. Arch. Biochem. Biophys. 293, 140–146. [DOI] [PubMed] [Google Scholar]
- Girod, A., Storrie, B., Simpson, J. C., Johannes, L., Goud, B., Roberts, L. M., Lord, J. M., Nilsson, T., and Pepperkok, R. (1999). Evidence for a COP-I-independent transport route from the Golgi complex to the endoplasmic reticulum. Nat. Cell Biol. 1, 423–430. [DOI] [PubMed] [Google Scholar]
- Gruenberg, J. (2001). The endocytic pathway: a mosaic of domains. Nat. Rev. Mol. Cell Biol. 2, 721–730. [DOI] [PubMed] [Google Scholar]
- Harder, T., Scheiffele, P., Verkade, P., and Simons, K. (1998). Lipid domain structure of the plasma membrane revealed by patching of membrane components. J. Cell Biol. 141, 929–942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazes, B., and Read, R. J. (1997). Accumulating evidence suggests that several AB-toxins subvert the endoplasmic reticulum-associated protein degradation pathway to enter target cells. Biochemistry 36, 11051–11054. [DOI] [PubMed] [Google Scholar]
- Helenius, A., and Aebi, M. (2004). Roles of N-linked glycans in the endoplasmic reticulum. Annu. Rev. Biochem. 73, 1019–1049. [DOI] [PubMed] [Google Scholar]
- Hoey, D. E., Sharp, L., Currie, C., Lingwood, C. A., Gally, D. L., and Smith, D. G. (2003). Verotoxin 1 binding to intestinal crypt epithelial cells results in localization to lysosomes and abrogation of toxicity. Cell Microbiol. 5, 85–97. [DOI] [PubMed] [Google Scholar]
- Hunnam, V., Harvey, D. J., Priestman, D. A., Bateman, R. H., Bordoli, R. S., and Tyldesley, R. (2001). Ionization and fragmentation of neutral and acidic glycosphingolipids with a Q-TOF mass spectrometer fitted with a MALDI ion source. J. Am. Soc. Mass Spectrom. 12, 1220–1225. [DOI] [PubMed] [Google Scholar]
- Ichikawa, S., Nakajo, N., Sakiyama, H., and Hirabayashi, Y. (1994). A mouse B16 melanoma mutant deficient in glycolipids. Proc. Natl. Acad. Sci. USA. 91, 2703–2707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inokuchi, J. I., Uemura, S., Kabayama, K., and Igarashi, Y. (2000). Glycosphingolipid deficiency affects functional microdomain formation in Lewis lung carcinoma cells. Glycoconj. J. 17, 239–245. [DOI] [PubMed] [Google Scholar]
- Jacewicz, M., Clausen, H., Nudelman, E., Donohue-Rolfe, A., and Keusch, G. T. (1986). Pathogenesis of shigella diarrhea. XI. Isolation of a shigella toxin-binding glycolipid from rabbit jejunum and HeLa cells and its identification as globotriaosylceramide. J. Exp. Med. 163, 1391–1404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johannes, L., Tenza, D., Antony, C., and Goud, B. (1997). Retrograde transport of KDEL-bearing B-fragment of Shiga toxin. J. Biol. Chem. 272, 19554–19561. [DOI] [PubMed] [Google Scholar]
- Kamhi-Nesher, S., Shenkman, M., Tolchinsky, S., Fromm, S. V., Ehrlich, R., and Lederkremer, G. Z. (2001). A novel quality control compartment derived from the endoplasmic reticulum. Mol. Biol. Cell 12, 1711–1723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kok, J. W., Babia, T., Filipeanu, C. M., Nelemans, A., Egea, G., and Hoekstra, D. (1998). PDMP blocks brefeldin A-induced retrograde membrane transport from Golgi to ER: evidence for involvement of calcium homeostasis and dissociation from sphingolipid metabolism. J. Cell Biol. 142, 25–38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lala, P., Ito, S., and Lingwood, C. A. (2000). Retroviral transfection of Madin-Darby canine kidney cells with human MDR1 results in a major increase in globotriaosylceramide and 10(5)- to 10(6)-fold increased cell sensitivity to verocytotoxin. Role of p-glycoprotein in glycolipid synthesis. J. Biol. Chem. 275, 6246–6251. [DOI] [PubMed] [Google Scholar]
- Landwojtowicz, E., Nervi, P., and Seelig, A. (2002). Real-time monitoring of P-glycoprotein activation in living cells. Biochemistry 41, 8050–8057. [DOI] [PubMed] [Google Scholar]
- LaPointe, P., Wei, X., and Gariepy, J. (2005). A role for the protease-sensitive loop region of Shiga-like toxin 1 in the retrotranslocation of its A1 domain from the endoplasmic reticulum lumen. J. Biol. Chem. 280, 23310–23318. [DOI] [PubMed] [Google Scholar]
- Lavie, Y., Cao, H., Volner, A., Lucci, A., Han, T. Y., Geffen, V., Giuliano, A. E., and Cabot, M. C. (1997). Agents that reverse multidrug resistance, tamoxifen, verapamil, and cyclosporin A, block glycosphingolipid metabolism by inhibiting ceramide glycosylation in human cancer cells. J. Biol. Chem. 272, 1682–1687. [DOI] [PubMed] [Google Scholar]
- Lencer, W. I., and Tsai, B. (2003). The intracellular voyage of cholera toxin: going retro. Trends Biochem. Sci. 28, 639–645. [DOI] [PubMed] [Google Scholar]
- Li, N., Mak, A., Richards, D. P., Naber, C., Keller, B. O., Li, L., and Shaw, A. R. (2003). Monocyte lipid rafts contain proteins implicated in vesicular trafficking and phagosome formation. Proteomics 3, 536–548. [DOI] [PubMed] [Google Scholar]
- Lilley, B. N., and Ploegh, H. L. (2004). A membrane protein required for dislocation of misfolded proteins from the ER. Nature 429, 834–840. [DOI] [PubMed] [Google Scholar]
- Ling, H., Boodhoo, A., Hazes, B., Cummings, M. D., Armstrong, G. D., Brunton, J. L., and Read, R. J. (1998). Structure of the shiga-like toxin I B-pentamer complexed with an analogue of its receptor Gb3. Biochemistry 37, 1777–1788. [DOI] [PubMed] [Google Scholar]
- Lisanti, M. P., Tang, Z., Scherer, P. E., and Sargiacomo, M. (1995). Caveolae purification and glycosylphosphatidylinositol-linked protein sorting in polarized epithelia. Methods Enzymol. 250, 655–668. [DOI] [PubMed] [Google Scholar]
- London, E., and Brown, D. A. (2000). Insolubility of lipids in Triton X-100, physical origin and relationship to sphingolipid/cholesterol membrane domains (rafts). Biochim. Biophys. Acta 1508, 182–195. [DOI] [PubMed] [Google Scholar]
- Lord, J. M., Deeks, E., Marsden, C. J., Moore, K., Pateman, C., Smith, D. C., Spooner, R. A., Watson, P., and Roberts, L. M. (2003). Retrograde transport of toxins across the endoplasmic reticulum membrane. Biochem. Soc. Trans. 31, 1260–1262. [DOI] [PubMed] [Google Scholar]
- Mallard, F., Antony, C., Tenza, D., Salamero, J., Goud, B., and Johannes, L. (1998). Direct pathway from early/recycling endosomes to the Golgi apparatus revealed through the study of shiga toxin B-fragment transport. J. Cell Biol. 143, 973–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallard, F., Tang, B. L., Galli, T., Tenza, D., Saint-Pol, A., Yue, X., Antony, C., Hong, W., Goud, B., and Johannes, L. (2002). Early/recycling endosomes-to-TGN transport involves two SNARE complexes and a Rab6 isoform. J. Cell Biol. 156, 653–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mancini, R., Aebi, M., and Helenius, A. (2003). Multiple endoplasmic reticulum-associated pathways degrade mutant yeast carboxypeptidase Y in mammalian cells. J. Biol. Chem. 278, 46895–46905. [DOI] [PubMed] [Google Scholar]
- Miller-Podraza, H., Mansson, J. E., and Svennerholm, L. (1992). Isolation of complex gangliosides from human brain. Biochim. Biophys. Acta 1124, 45–51. [DOI] [PubMed] [Google Scholar]
- Neville, D. C., Coquard, V., Priestman, D. A., te Vruchte, D. J., Sillence, D. J., Dwek, R. A., Platt, F. M., and Butters, T. D. (2004). Analysis of fluorescently labeled glycosphingolipid-derived oligosaccharides following ceramide glycanase digestion and anthranilic acid labeling. Anal. Biochem. 331, 275–282. [DOI] [PubMed] [Google Scholar]
- Nichols, B. J., Kenworthy, A. K., Polishchuk, R. S., Lodge, R., Roberts, T. H., Hirschberg, K., Phair, R. D., and Lippincott-Schwartz, J. (2001). Rapid cycling of lipid raft markers between the cell surface and Golgi complex. J. Cell Biol. 153, 529–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parkin, E. T., Turner, A. J., and Hooper, N. M. (2001). Differential effects of glycosphingolipidsonthedetergent-insolubilityoftheglycosylphosphatidylinositol-anchored membrane dipeptidase. Biochem. J. 358, 209–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pike, L. J., Han, X., Chung, K. N., and Gross, R. W. (2002). Lipid rafts are enriched in arachidonic acid and plasmenylethanolamine and their composition is independent of caveolin-1 expression: a quantitative electrospray ionization/mass spectrometric analysis. Biochemistry 41, 2075–2088. [DOI] [PubMed] [Google Scholar]
- Platt, F. M., Neises, G. R., Karlsson, G. B., Dwek, R. A., and Butters, T. D. (1994). N-Butyldeoxygalactonojirimycin inhibits glycolipid biosynthesis but does not affect N-linked oligosaccharide processing. J. Biol. Chem. 269, 27108–27114. [PubMed] [Google Scholar]
- Pralle, A., Keller, P., Florin, E. L., Simons, K., and Horber, J. K. (2000). Sphingolipid-cholesterol rafts diffuse as small entities in the plasma membrane of mammalian cells. J. Cell Biol. 148, 997–1008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pudymaitis, A., Armstrong, G., and Lingwood, C. A. (1991). Verotoxin-resistant cell clones are deficient in the glycolipid globotriosylceramide: differential basis of phenotype. Arch. Biochem. Biophys. 286, 448–452. [DOI] [PubMed] [Google Scholar]
- Puri, V., Watanabe, R., Singh, R. D., Dominguez, M., Brown, J. C., Wheatley, C. L., Marks, D. L., and Pagano, R. E. (2001). Clathrin-dependent and -independent internalization of plasma membrane sphingolipids initiates two Golgi targeting pathways. J. Cell Biol. 154, 535–547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts, L. M., and Smith, D. C. (2004). Ricin: the endoplasmic reticulum connection. Toxicon 44, 469–472. [DOI] [PubMed] [Google Scholar]
- Rodighiero, C., Fujinaga, Y., Hirst, T. R., and Lencer, W. I. (2001). A cholera toxin B-subunit variant that binds ganglioside GM1 but fails to induce toxicity. J. Biol. Chem. 276, 36939–36945. [DOI] [PubMed] [Google Scholar]
- Rodighiero, C., Tsai, B., Rapoport, T. A., and Lencer, W. I. (2002). Role of ubiquitination in retro-translocation of cholera toxin and escape of cytosolic degradation. EMBO Rep. 3, 1222–1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K., Ryd, M., Garred, O., Schweda, E., Holm, P. K., and van Deurs, B. (1994). Retrograde transport from the Golgi complex to the ER of both Shiga toxin and the nontoxic Shiga B-fragment is regulated by butyric acid and cAMP. J. Cell Biol. 126, 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandvig, K., Spilsberg, B., Lauvrak, S. U., Torgersen, M. L., Iversen, T. G., and van Deurs, B. (2004). Pathways followed by protein toxins into cells. Int. J. Med. Microbiol. 293, 483–490. [DOI] [PubMed] [Google Scholar]
- Sandvig, K., and van Deurs, B. (2002). Membrane traffic exploited by protein toxins. Annu. Rev. Cell Dev. Biol. 18, 1–24. [DOI] [PubMed] [Google Scholar]
- Schutz, G. J., Kada, G., Pastushenko, V. P., and Schindler, H. (2000). Properties of lipid microdomains in a muscle cell membrane visualized by single molecule microscopy. EMBO J. 19, 892–901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sevlever, D., Pickett, S., Mann, K. J., Sambamurti, K., Medof, M. E., and Rosenberry, T. L. (1999). Glycosylphosphatidylinositol-anchor intermediates associate with triton-insoluble membranes in subcellular compartments that include the endoplasmic reticulum. Biochem. J. 343, 627–635. [PMC free article] [PubMed] [Google Scholar]
- Sharma, D. K., Choudhury, A., Singh, R. D., Wheatley, C. L., Marks, D. L., and Pagano, R. E. (2003). Glycosphingolipids internalized via caveolar-related endocytosis rapidly merge with the clathrin pathway in early endosomes and form microdomains for recycling. J. Biol. Chem. 278, 7564–7572. [DOI] [PubMed] [Google Scholar]
- Shu, L., Lee, L., Chang, Y., Holzman, L. B., Edwards, C. A., Shelden, E., and Shayman, J. A. (2000). Caveolar structure and protein sorting are maintained in NIH 3T3 cells independent of glycosphingolipid depletion. Arch. Biochem. Biophys. 373, 83–90. [DOI] [PubMed] [Google Scholar]
- Sillence, D. J., Puri, V., Marks, D. L., Butters, T. D., Dwek, R. A., Pagano, R. E., and Platt, F. M. (2002). Glucosylceramide modulates membrane traffic along the endocytic pathway. J. Lipid Res. 43, 1837–1845. [DOI] [PubMed] [Google Scholar]
- Sillence, D. J., Raggers, R. J., Neville, D. C., Harvey, D. J., and van Meer, G. (2000). Assay for the transbilayer distribution of glycolipids. Selective oxidation of glucosylceramide to glucuronylceramide by TEMPO nitroxyl radicals. J. Lipid Res. 41, 1252–1260. [PubMed] [Google Scholar]
- Simons, K., and Ikonen, E. (1997). Functional rafts in cell membranes. Nature 387, 569–572. [DOI] [PubMed] [Google Scholar]
- Simons, K., and Toomre, D. (2000). Lipid rafts and signal transduction. Nat. Rev. Mol. Cell Biol. 1, 31–39. [DOI] [PubMed] [Google Scholar]
- Simons, M., Friedrichson, T., Schulz, J. B., Pitto, M., Masserini, M., and Kurzchalia, T. V. (1999). Exogenous administration of gangliosides displaces GPI-anchored proteins from lipid microdomains in living cells. Mol. Biol. Cell 10, 3187–3196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson, J. C., Roberts, L. M., Romisch, K., Davey, J., Wolf, D. H., and Lord, J. M. (1999). Ricin A chain utilises the endoplasmic reticulum-associated protein degradation pathway to enter the cytosol of yeast. FEBS Lett. 459, 80–84. [DOI] [PubMed] [Google Scholar]
- Smith, D. C., Lord, J. M., Roberts, L. M., and Johannes, L. (2004). Glycosphingolipids as toxin receptors. Semin. Cell Dev. Biol. 15, 397–408. [DOI] [PubMed] [Google Scholar]
- Smith, D. C., Marsden, C. J., Lord, J. M., and Roberts, L. M. (2003). Expression, purification and characterization of ricin vectors used for exogenous antigen delivery into the MHC class I presentation pathway. Biol. Proced. Online 5, 13–19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spooner, R. A., Watson, P. D., Marsden, C. J., Smith, D. C., Moore, K. A., Cook, J. P., Lord, J. M., and Roberts, L. M. (2004). Protein disulphide-isomerase reduces ricin to its A and B chains in the endoplasmic reticulum. Biochem. J. 383, 285–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suhan, M. L., and Hovde, C. J. (1998). Disruption of an internal membrane-spanning region in Shiga toxin 1 reduces cytotoxicity. Infect. Immun. 66, 5252–5259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter, K., Allyn, R. L., Jobling, M. G., and Holmes, R. K. (2002). Transfer of the cholera toxin A1 polypeptide from the endoplasmic reticulum to the cytosol is a rapid process facilitated by the endoplasmic reticulum-associated degradation pathway. Infect. Immun. 70, 6166–6171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter, K., Jobling, M. G., and Holmes, R. K. (2003). A class of mutant CHO cells resistant to cholera toxin rapidly degrades the catalytic polypeptide of cholera toxin and exhibits increased endoplasmic reticulum-associated degradation. Traffic 4, 232–242. [DOI] [PubMed] [Google Scholar]
- Tsai, B., and Rapoport, T. A. (2002). Unfolded cholera toxin is transferred to the ER membrane and released from protein disulfide isomerase upon oxidation by Ero1. J. Cell Biol. 159, 207–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai, B., Rodighiero, C., Lencer, W. I., and Rapoport, T. A. (2001). Protein disulfide isomerase acts as a redox-dependent chaperone to unfold cholera toxin. Cell 104, 937–948. [DOI] [PubMed] [Google Scholar]
- van Echten, G., Iber, H., Stotz, H., Takatsuki, A., and Sandhoff, K. (1990). Uncoupling of ganglioside biosynthesis by brefeldin A. Eur. J. Cell Biol. 51, 135–139. [PubMed] [Google Scholar]
- Vance, D. E., and Sweeley, C. C. (1967). Quantitative determination of the neutral glycosyl ceramides in human blood. J. Lipid Res. 8, 621–630. [PubMed] [Google Scholar]
- Varma, R., and Mayor, S. (1998). GPI-anchored proteins are organized in submicron domains at the cell surface. Nature 394, 798–801. [DOI] [PubMed] [Google Scholar]
- Wesche, J., Rapak, A., and Olsnes, S. (1999). Dependence of ricin toxicity on translocation of the toxin A-chain from the endoplasmic reticulum to the cytosol. J. Biol. Chem. 274, 34443–34449. [DOI] [PubMed] [Google Scholar]
- White, J., et al. (1999). Rab6 coordinates a novel Golgi to ER retrograde transport pathway in live cells. J. Cell Biol. 147, 743–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson, B. S., Pfeiffer, J. R., and Oliver, J. M. (2000). Observing FcepsilonRI signaling from the inside of the mast cell membrane. J. Cell Biol. 149, 1131–1142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wing, D. R., Garner, B., Hunnam, V., Reinkensmeier, G., Andersson, U., Harvey, D. J., Dwek, R. A., Platt, F. M., and Butters, T. D. (2001). High-performance liquid chromatography analysis of ganglioside carbohydrates at the picomole level after ceramide glycanase digestion and fluorescent labeling with 2-aminobenzamide. Anal. Biochem. 298, 207–217. [DOI] [PubMed] [Google Scholar]
- Ye, Y., Shibata, Y., Yun, C., Ron, D., and Rapoport, T. A. (2004). A membrane protein complex mediates retro-translocation from the ER lumen into the cytosol. Nature 429, 841–847. [DOI] [PubMed] [Google Scholar]
- Yegneswaran, S., Deguchi, H., and Griffin, J. H. (2003). Glucosylceramide, a neutral glycosphingolipid anticoagulant cofactor, enhances the interaction of human- and bovine-activated protein C with negatively charged phospholipid vesicles. J. Biol. Chem. 278, 14614–14621. [DOI] [PubMed] [Google Scholar]
- Yu, M., and Haslam, D. B. (2005). Shiga toxin is transported from the endoplasmic reticulum following interaction with the luminal chaperone HEDJ/ERdj3. Infect. Immun. 73, 2524–2532. [DOI] [PMC free article] [PubMed] [Google Scholar]