Abstract
Mitochondria consist of four compartments–outer membrane, intermembrane space, inner membrane, and matrix—with crucial but distinct functions for numerous cellular processes. A comprehensive characterization of the proteome of an individual mitochondrial compartment has not been reported so far. We used a eukaryotic model organism, the yeast Saccharomyces cerevisiae, to determine the proteome of highly purified mitochondrial outer membranes. We obtained a coverage of ∼85% based on the known outer membrane proteins. The proteome represents a rich source for the analysis of new functions of the outer membrane, including the yeast homologue (Hfd1/Ymr110c) of the human protein causing Sjögren–Larsson syndrome. Surprisingly, a subclass of proteins known to reside in internal mitochondrial compartments were found in the outer membrane proteome. These seemingly mislocalized proteins included most top scorers of a recent genome-wide analysis for mRNAs that were targeted to mitochondria and coded for proteins of prokaryotic origin. Together with the enrichment of the precursor form of a matrix protein in the outer membrane, we conclude that the mitochondrial outer membrane not only contains resident proteins but also accumulates a conserved subclass of preproteins destined for internal mitochondrial compartments.
INTRODUCTION
Mitochondria are crucial for numerous functions of virtually every eukaryotic cell, including bioenergetics, apoptosis, and metabolism of amino acids, lipids, and iron (Neupert, 1997; Scheffler, 1999; Newmeyer and Ferguson-Miller, 2003; Green and Kroemer, 2004; Lill and Mühlenhoff, 2005). Many diseases have been linked to mitochondrial dysfunction (Schapira, 2000; Schon, 2000; Steinmetz et al., 2002; Wallace, 2005). To date, the most comprehensive proteomic analysis of an entire cell organelle has been performed for mitochondria (Mootha et al., 2003; Sickmann et al., 2003; Taylor et al., 2003; Prokisch et al., 2004). In the yeast S. cerevisiae, a coverage of ∼80% of the mitochondrial proteome was achieved (Sickmann et al., 2003; Jensen et al., 2004; Prokisch et al., 2004; Reichert and Neupert, 2004). These studies indicate that yeast mitochondria contain up to 1000 different proteins, i.e., ∼17% of the ∼6000 different proteins synthesized in a yeast cell. Because yeast mitochondria synthesize only eight stable proteins encoded by the mitochondrial DNA in their matrix, 99% of all mitochondrial proteins are encoded by nuclear genes and synthesized as precursor proteins on cytosolic ribosomes. These precursor proteins are typically posttranslationally imported into mitochondria and sorted into one of the four mitochondrial compartments: outer membrane, intermembrane space, inner membrane, and matrix (Neupert, 1997; Jensen and Dunn, 2002; Endo et al., 2003; Mihara, 2003; Truscott et al., 2003; Koehler, 2004). Most preproteins destined for the mitochondrial matrix and several proteins destined for inner membrane or intermembrane space contain amino-terminal presequences that function as targeting signals.
Each of the four mitochondrial compartments harbors specific functions and structures, e.g., the enzymes of the citric acid cycle are located in the matrix, the respiratory chain complexes are present in the inner membrane, factors released from mitochondria during programmed cell death originate from the intermembrane space, and the general entry gate for precursor proteins (translocase of outer membrane [TOM] complex) and the sorting and assembly machinery (SAM) complex are embedded in the outer membrane (Neupert, 1997; Scheffler, 1999; Newmeyer and Ferguson-Miller, 2003; Wiedemann et al., 2003; Pfanner et al., 2004). A systematic proteomic characterization of the individual compartments will be of high importance for a molecular understanding of mitochondrial functions. Da Cruz et al. (2003) performed a partial proteomic analysis of enriched mitochondrial inner membranes from mouse liver. However, no comprehensive analysis of an individual mitochondrial compartment has been reported so far; thus, our knowledge about the composition and functional relevance of the mitochondrial compartments is limited.
Here, we performed a comprehensive proteomic analysis of the outer membrane of yeast mitochondria. Our experimental strategy involved the following steps. 1) Because a high purity of an isolated mitochondrial compartment is an essential prerequisite for a proteomic analysis, we selected outer membrane vesicles, which of all four mitochondrial compartments of S. cerevisiae are best accessible to a selective purification (Alconada et al., 1995; van Wilpe et al., 1999; Meisinger et al., 2001). Protocols for high-purity isolation of mitochondria and outer membrane vesicles were combined in a successive manner to yield pure outer membranes. 2) To avoid the limitations of individual separation and detection methods, we used several different approaches for separation of proteins and tryptic peptides as well as for the analysis by mass spectrometry, in parallel. 3) Unexpected protein localization results were subjected to an integrative analysis by comparison to a genome-wide study of mRNA targeting to mitochondria and evolutionary relationships of mitochondrial proteins (Marc et al., 2002). These combined approaches yielded important new insight into mitochondrial dynamics and biogenesis.
MATERIALS AND METHODS
Isolation of Mitochondrial Outer Membrane Vesicles
Yeast wild-type strain YPH499 (Sikorski and Hieter, 1989) was grown in YPG medium (1% (wt/vol) yeast extract, 2% (wt/vol) bactopeptone, and 3% (wt/vol) glycerol) at 30°C until an optical density of 2. A crude mitochondrial fraction (P12) was obtained by differential centrifugation as described previously (Daum et al., 1982; Meisinger et al., 2000). This fraction was further purified via two three-step sucrose gradients, yielding highly pure mitochondria (Meisinger et al., 2000; Sickmann et al., 2003). For isolation of outer membranes, purified mitochondria (100 mg of protein) were diluted in swelling buffer (5 mM potassium phosphate, pH 7.4, and 1 mM phenylmethylsulfonyl fluoride [PMSF]) and incubated on ice for 20 min at a protein concentration of 2–4 mg/ml. After treatment with a glass-Teflon potter (15–20 strokes), the homogenate was loaded on top of a discontinuous sucrose gradient [1 ml 60%, 4 ml 32%, 1 ml 15% (wt/vol) sucrose] in EM buffer [10 mM 3-(N-morpholino)propanesulfonic acid MOPS, pH 7.2, and 1 mM EDTA)]. After centrifugation in a swing-out rotor (1 h; 134,000 × g; 2°C), outer membranes were recovered from the 15–32% interface, and the sucrose concentration was adjusted to 50% with a 70% (wt/vol) sucrose/EM solution. This sample was then loaded onto the bottom of a centrifuge tube and overlaid with 5 ml of 32% (wt/vol) sucrose/EM and 1.5 ml of EM buffer. After flotation at 240,000 × g at 2°C overnight, purified outer membrane vesicles were collected from the 0–32% interface, diluted fivefold with EM buffer, and pelleted via centrifugation at 160,000 × g for 30 min (Alconada et al., 1995; Meisinger et al., 2001). Membranes were resuspended in EM buffer and stored in aliquots at –80°C.
Separation of Outer and Inner Membrane Vesicles
Mitochondrial pellets (2–5 mg of protein) were resuspended in 20 mM HEPES/KOH, pH 7.4, 0.5 mM EDTA, and 1 mM PMSF and incubated for 30 min on ice at a concentration of 1 mg/ml. After adjustment to a final sucrose concentration of 0.45 M and further incubation for 10 min on ice, the sample was sonified with a Branson Sonifier 250 (duty cycle 80%) for 90 s. After a clarifying spin at 20,000 × g for 15 min, the supernatant was centrifuged at 200,000 × g for 45 min. The pellet, containing membrane vesicles, was resuspended in 400 μl of loading buffer (5 mM HEPES/KOH, pH 7.4, 10 mM KCl, and 1 mM PMSF) and layered on top of a discontinuous sucrose gradient (1.5 ml of 1.6 M sucrose, 5.5 ml of 1.35 M sucrose, 2.5 ml of 1.1 M sucrose, and 1.5 ml of 0.85 M sucrose in 5 mM HEPES/KOH, pH 7.4, and 10 mM KCl). After centrifugation at 134,000 × g and 2°C for 16 h, fractions (750 μl) were collected and analyzed by SDS-PAGE and Western blotting using polyvinylidene diflouride (PVDF) membranes (Millipore, Billerica, MA) and the enhanced chemiluminescence system (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). Quantification of immunoreactive bands was performed with the NIH Image program.
Mitochondrial Surface Fraction
Purified mitochondria were incubated at a protein concentration of 1 mg/ml in SEM buffer (10 mM MOPS, pH 7.2, 250 mM sucrose, 1 mM EDTA) containing 20 μg/ml trypsin for 15 min on ice. After centrifugation for 15 min at 20,000 × g, the supernatant, containing the mitochondrial surface fraction, was separated by SDS-PAGE. Gel slices (1 mm) were excised and subjected to in-gel digestion followed by mass spectrometry based peptide identification as described for two-dimensional benzyldimethyl-n-hexadecylammonium chloride (2D-BAC)/SDS-PAGE (see below).
2D-BAC/SDS-PAGE
Outer membrane vesicles were centrifuged in EM buffer at 100,000 × g and 4°C for 1 h. Pellets were resuspended in 5 μl of 1× BAC sample buffer (3.75 M urea, 125 mM BAC, 5% glycerol, 0.025% pyronin Y, and 60 mM dithiothreitol [DTT]) and incubated at 60°C for 10 min. First dimensions were carried out either on 10, 12, or 15% slab gels overlaid with 4% stacking gels (Zahedi et al., 2005) for 20 min at 4°C and 30 mA/gel followed by 60 min at 60 mA/gel. Afterward, whole sample lanes were excised, equilibrated three times for 10 min with 100 mM Tris-HCl, pH 6.8, and incubated in 3× SDS sample buffer containing 60 mM DTT for 10 min. Lanes were transferred on 4–20, 10, or 12% Tris-glycine SDS gels. Second dimensions were carried out at 4°C for 15 min at 15 mA/gel and then for 40 min at 50 mA/gel. Subsequently, gels were stained according to Shevchenko et al. (1996). Visualized protein spots were excised. Washing of spots and in-gel digestion were accomplished according to Shevchenko et al. (1996) with slight modifications. Dried protein spots were incubated with 12.5 ng/μl trypsin (Sequencing grade modified; Promega, Madison, WI) in 50 mM NH4HCO3. Digestion was carried out for 8 h at 37°C.
Peptides were extracted, depending on the following mass spectrometric (MS) method, in a three-step (nano-liquid chromatography-tandem mass spectrometry [LC-MS/MS]) or in a two-step (matrix-assisted laser desorption ionization [MALDI]-MS) procedure. For nano-LC-MS/MS analysis, peptides were extracted twice with 10 μl of 5% formic acid (FA) each and once with 10 μl of 5% FA, 50% acetonitrile (ACN). Extracts were combined, and the organic content was reduced under vacuum to improve compatibility to reversedphase chromatography. For MALDI-MS, peptides were extracted once with 10 μl of 0.1% trifluoroacetic acid, concentrated using μC18 ZipTips (Millipore) according to the manufacturer's instructions, and eluted directly onto a MTP 384 polished steel target plate (Bruker Daltonik, Bremen, Germany).
Multidimensional Liquid Chromatography (MDLC)
Outer membrane vesicles in 20 μl of EM buffer were digested using 0.5 μg of trypsin (5000 U/mg) in 20 μlof25 mM NH4HCO3 for 8 h at 37°C. The sample volume was reduced to 6 μl in a vacuum centrifuge, and the solution was acidified using 5% FA. In the first dimension, the tryptic peptide mixture was separated by strong cation exchange chromatography (PL-SCX; 300-μm inner diameter [ID], 150-mm length, 1000-Å pore size, 8-μm particle size, custom packed) using a linear binary gradient (solvent A: 20 mM KH2PO4, pH 3.0; solvent B: 20 mM KH2PO4, 0.25 mM NaCl, 25% ACN, pH 5.5). Although increasing the overall peptide recovery, the use of an organic modifier renders online nano-LC-MS/MS coupling impossible (Wagner et al., 2003). Therefore, minute-by-minute fractions were collected, the organic solvent was removed under vacuum, and each sample was analyzed in the second dimension by nano-LC-MS/MS separately.
Nano-LC-MS/MS
Nano-LC-MS/MS analyses were accomplished either on a LCQ Deca XPplus (Thermo Electron, Dreieich, Germany), a Qstar XL (Applied Biosystems, Darmstadt, Germany), or a Qtrap 4000 (Applied Biosystems) mass spectrometer directly coupled to an UltiMate nano-LC system (Dionex, Idstein, Germany). Samples were online-desalted and preconcentrated using a C18 PepMap trapping column (300-μm ID, 1-mm length, 100-Å pore size, 5-μm particle size; Dionex) and subsequently separated on a C18 PepMap main column (75-μm ID, 150-mm length, 100-Å pore size, 3-μm particle size; Dionex) using a linear binary gradient (solvent A: 0.1% FA; solvent B: 0.1% FA, 84% ACN) at a flow rate of 250 nl/min according to Mitulovic et al. (2003, 2004). Full MS scans from 400 to 1600 m/z were recorded, and the four (LCQ Deca XPplus, Qtrap 4000) or three (Qstar XL) most intensive peptide ions were subjected to further fragmentation considering a dynamic exclusion.
MALDI-Time of Flight (TOF)-MS/MS
MALDI-time of flight analyses were accomplished on an Ultraflex TOF/TOF (Bruker Daltonik) in reflector mode after external calibration using a custom-made peptide standard. Additionally, acquired spectra were internally calibrated using tryptic autoproteolysis signals at 842.5094 m/z, 2211.104 m/z, and 2283.180 m/z.
Data Interpretation
To exclude consideration of false positive protein hits derived from search algorithms, only proteins, which were identified by both MASCOT as well as SEQUEST and of which at least two independent fragment ion spectra were validated manually, were taken into account. An in-house software solution named resDB was used for filtering, grouping, and viewing the Mascot result files obtained from the MS/MS data. resDB is a database based solution running on the relational database system PostgreSQL featuring import and export of MS/MS result files, including a spectra archive. The user application features viewing single results as well as grouping of one or more MDLC runs that usually yield Mascot result files of more than half a gigabyte in size, which cannot be handled by the viewer coming with Mascot. For internal peptide hit scoring, we normalized each single Mascot score with its corresponding significance threshold. Grouped protein hits were scored according to the count of unique peptides, spectra per peptide and exact protein sequence coverage. The application additionally features the presentation of peptide results, including the corresponding MS/MS spectrum and the theoretical peptide spectrum as well as a direct link to our internal protein sequence database seqDB that presents sequence coverage and peptide locations.
The significance of a single peptide hit obtained by using Mascot can be calculated from the Mascot score Sm and the internal significance p in conjunction with the corresponding score threshold sp according to the following equations:
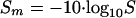 |
(1) |
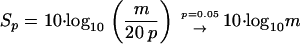 |
(2) |
with m being the average of the internal scores S of a single Mascot run.
Given another significance level y, a new threshold Sy can be calculated according to
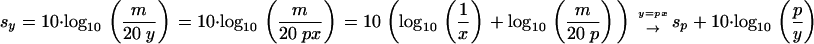 |
(3) |
Therefore, the significance level of a single Mascot score S = sy can be calculated as follows, if the original threshold sp and significance p are available–the default value of p is 0.05.
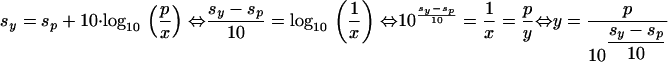 |
(4) |
According to Eq. 4, we calculated the significance of each peptide hit as the probability for a false positive.
For generation of a complete peptide list and calculation of the sequence coverage, only peptides with a Mascot score above the threshold sp+ 10 (MLDC and 2D-BAC/SDS-PAGE samples) were taken into account, corresponding to a probability of 0.005 for a false positive. Molecular weights and isoelectric points were calculated (Skoog and Wichmann, 1986) according to the sequences deposited in the Saccharomyces Genome Database (Cherry et al., 1997).
RESULTS
Identification of Proteins in Purified Mitochondrial Outer Membrane Vesicles
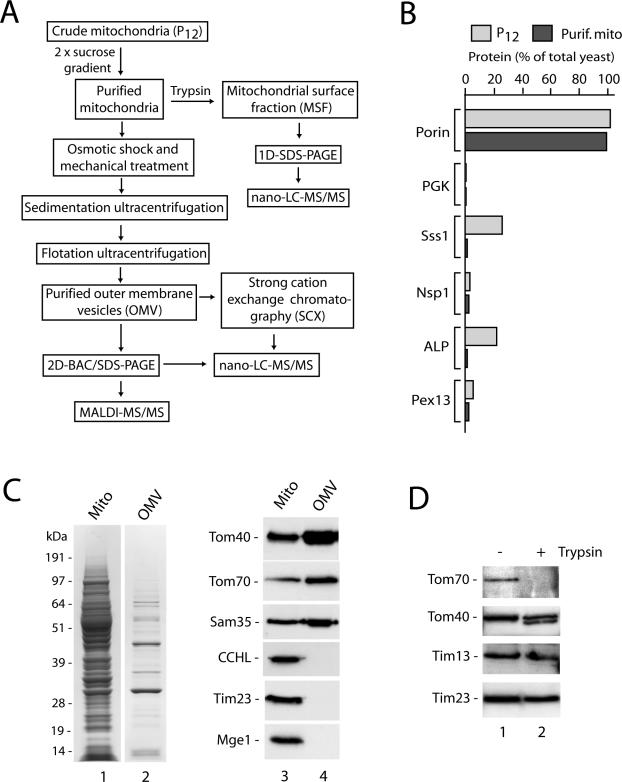
The experimental strategy is outlined in Figure 1A. S. cerevisiae mitochondria were isolated by differential centrifugation. This crude mitochondrial fraction was virtually free of the cytosolic marker protein phosphoglycerate kinase and the nuclear marker Nsp1, but it contained significant contaminations from the endoplasmic reticulum (Sss1), vacuole (alkaline phosphatase [ALP]), and a small fraction of peroxisomes (Pex13) (Figure 1B) (Meisinger et al., 2000; Sickmann et al., 2003). By applying two successive sucrose gradients, highly pure mitochondria were obtained that were also virtually free of the marker proteins for the endoplasmic reticulum, vacuole, and peroxisomes (Figure 1B) (Sickmann et al., 2003). By gentle mechanical forces applied to the mitochondria, outer membrane vesicles were sheared off and separated from the remaining mitochondrial compartments by successive sedimentation and flotation steps. The resulting outer membrane vesicles (Figure 1C, lane 2) were devoid of marker proteins for the intermembrane space, inner membrane, and matrix (Figure 1C, lane 4). In addition, we prepared a mitochondrial surface fraction (MSF) by a treatment of isolated mitochondria with trypsin (Sickmann et al., 2003). The trypsin treatment degraded the surface-exposed receptor Tom70 but did not disrupt the outer membrane barrier as indicated by a limited clipping of the outer membrane-integrated channel Tom40 (Hill et al., 1998) and the protection of the intermembrane space-exposed proteins translocase of inner mitochondrial membrane (Tim) 13 and Tim23 (Figure 1D).
Figure 1.
Experimental strategy and purity of mitochondrial fractions from S. cerevisiae. (A) Flow chart showing purification of mitochondrial outer membranes and mitochondrial surface fraction followed by MS analysis. (B) Isolation of highly purified mitochondria. Crude mitochondrial fractions (P12) were purified twice via sucrose gradients, yielding purified mitochondria essentially devoid of marker proteins from other cell compartments. Equal-volume aliquots from mitochondria and total yeast extracts were separated by SDS-PAGE, followed by Western blotting and immunodecoration. Immunoreactive bands were quantified and the value for total yeast extract of each protein was set to 100%. Porin, mitochondrial outer membrane protein; PGK, phosphoglycerate kinase (cytosol); Sss1, subunit of Sec61 translocation complex (endoplasmic reticulum); Nsp1, subunit of nuclear pore complex; ALP, vacuolar ALP; Pex13, peroxin13. (C) Purification of outer membrane vesicles. Purified mitochondria and outer membrane vesicles were separated by SDS-PAGE and then either stained with Coomassie brilliant blue (lanes 1 and 2) or transferred onto PVDF membranes followed by immunodecoration (lanes 3 and 4). Tom40, Tom70, subunits of the preprotein translocase of the outer membrane; Sam35, subunit of the sorting and assembly machinery of the outer membrane; CCHL, cytochrome c heme lyase of the intermembrane space; Tim23, subunit of the presequence translocase of the inner membrane; Mge1, soluble matrix protein. (D) Generation of the MSF. Highly purified mitochondria were incubated with or without trypsin (20 μg/ml) in SEM buffer for 15 min on ice. After pelleting, the mitochondria were separated by SDS-PAGE and analyzed by Western blotting and immunodecoration, whereas the supernatant (MSF) was subjected to nano-LC-MS/MS analysis (Supplemental Table 4).
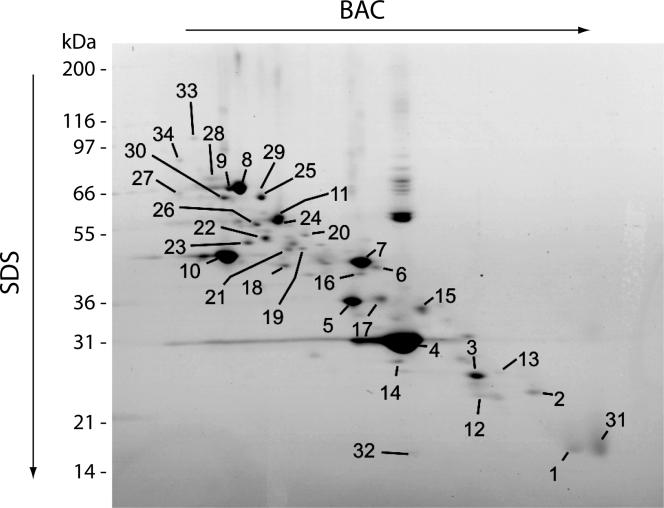
Although 2D-gel electrophoresis has been widely used for proteomic analysis, many proteins, in particular membrane proteins, escape detection by this method. For example, in the comprehensive proteomic analysis of yeast mitochondria with 749 identified different proteins, only 109 different proteins (15%) were identified by an extensive 2D-analysis (Sickmann et al., 2003). We thus used a 2D-analysis optimized for membrane proteins with the detergent BAC in the first dimension and SDS in the second dimension (Zahedi et al., 2005). By nano-LC-MS/MS and MALDI-MS/MS, we identified 36 different proteins from purified outer membrane vesicles (Figure 2 and Supplemental Table 1). However, only 31% of the known outer membrane proteins were identified by this approach.
Figure 2.
2D-gel analysis of purified mitochondrial outer membranes. Purified outer membranes (∼30 μg of protein) of S. cerevisiae mitochondria were separated by BAC-PAGE in the first dimension followed by SDS-PAGE in the second dimension. Coomassie-stained spots were excised and analyzed either by nano-LC-MS/MS or MALDI-MS/MS as described in Materials and Methods. Thirty-six different proteins were identified and listed in Supplemental Table 1.
We thus subjected purified outer membranes to an extensive treatment with trypsin and separated the peptide mixture by SCX-chromatography. Nano-LC-MS/MS led to the identification of 112 different proteins (Table 1) with a coverage of ∼85% of all known mitochondrial outer membrane proteins, including integral and peripheral membrane proteins (Supplemental Table 2). In comparison to our previous analysis of the mitochondrial proteome (Sickmann et al., 2003), 27 additional proteins were identified (Supplemental Table 3). The additional proteins were mostly of low abundance (Ghaemmaghami et al., 2003), including Mdm10, Mdm34, Ubp16, and three subunits of the tRNA splicing endonuclease complex (Sen2, Sen34, and Sen54) (Yoshihisa et al., 2003), indicating a high sensitivity of the detection system applied.
Table 1.
List of all proteins identified in the mitochondrial outer membrane fraction separated by strong cation exchange chromatography (SCX) followed by reversed phase chromatography coupled to ESI-MS (nano-LC-MS/MS)
| Gene | Protein | ORF | Sequence coverage (%) | Predicted molecular mass (kDa) | Predicted isoelectric point | MSF | MLR | 2D-BAC/SDS-PAGE |
|---|---|---|---|---|---|---|---|---|
| ATP2 | F1F0-ATPase complex, F1β subunit | YJR121w | 42 | 54.7 | 5.5 | X | 98.2 | X |
| PDB1 | Pyruvate dehydrogenase (lipoamide) β chain precursor | YBR221c | 17 | 40.0 | 5.2 | X | 97.5 | |
| COR1 | Ubiquinol-cytochrome c reductase 44K core protein | YBL045c | 14 | 50.2 | 6.8 | X | 96.8 | X |
| ALD4 | Mitochondrial aldehyde dehydrogenase | YOR374w | 45 | 56.7 | 6.3 | X | 96.2 | |
| NDI1 | NADH-ubiquinone-6 oxidoreductase (homologue to human outer membrane protein AMID) | YML120c | 6 | 57.2 | 9.4 | 96.0 | ||
| MDH1 | Malate dehydrogenase, mitochondrial | YKL085w | 34 | 35.6 | 8.5 | X | 95.2 | |
| ATP3 | F1F0-ATPase complex, F1γ subunit | YBR039w | 32 | 34.3 | 9.3 | X | 95.0 | |
| MCR1 | Cytochrome b5 reductase | YKL150w | 64 | 34.1 | 8.7 | X | 95.0 | X |
| PDA1 | Pyruvate dehydrogenase complex E1-α subunit | YER178w | 23 | 46.3 | 8.3 | X | 95.0 | |
| SDH2 | Succinate dehydrogenase iron—sulfur protein subunit | YLL041c | 18 | 30.2 | 9.1 | 94.8 | ||
| VPS1 | Member of the dynamin family of GTPases | YKR001c | 25 | 78.7 | 7.7 | 94.0 | ||
| MIA40 | Essential mitochondrial protein required for import and assembly of intermembrane space proteins | YKL195w | 6 | 44.5 | 4.5 | X | 92.0 | |
| STE24 | Zinc metallo-protease | YJR117w | 18 | 52.3 | 7.7 | 91.7 | ||
| HSP60 | Heat-shock protein-chaperone, mitochondrial | YLR259c | 42 | 60.7 | 5.2 | X | 90.0 | |
| TOM70 | Mitochondrial outer membrane import receptor subunit | YNL121c | 57 | 70.0 | 5.2 | X | 90.0 | X |
| KGD1 | 2-Oxoglutarate dehydrogenase complex E1 component | YIL125w | 27 | 114.3 | 6.8 | X | 89.4 | X |
| GUT2 | Glycerol-3-phosphate dehydrogenase, mitochondrial | YIL155c | 48 | 72.3 | 8.0 | X | 89.4 | X |
| CYB2 | Lactate dehydrogenase cytochrome b2 | YML054c | 14 | 65.5 | 8.6 | X | 89.0 | X |
| FAA1 | Long chain fatty acid-CoA ligase | YOR317w | 47 | 77.8 | 7.5 | 89.0 | X | |
| OM45 | Protein of the outer mitochondrial membrane | YIL136w | 66 | 44.5 | 8.6 | X | 88.6 | X |
| SDH1 | Succinate dehydrogenase flavoprotein | YKL148c | 20 | 70.2 | 7.1 | 87.8 | X | |
| NCP1 | NADPH-cytochrome P450 reductase | YHR042w | 3 | 76.7 | 5.0 | 87.0 | ||
| GSF2 | Protein involved in glucose repression | YML048w | 7 | 45.8 | 6.6 | 87.0 | ||
| AFG1 | Member of the AFG1-like ATPase family | YEL052w | 6 | 58.3 | 8.7 | 86.0 | ||
| COR2 | Ubiquinol-cytochrome c reductase 40-kDa chain II | YPR191w | 35 | 40.4 | 7.8 | X | 83.8 | X |
| TIM44 | Subunit of the presequence translocase associated motor complex (PAM complex) | YIL022w | 16 | 48.8 | 9.4 | 83.0 | ||
| ILV5 | Ketol-acid reducto-isomerase | YLR355c | 19 | 44.3 | 9.1 | X | 82.7 | |
| BNA4 | Kynurenine 3-mono oxygenase | YBL098w | 33 | 52.4 | 8.6 | 82.0 | X | |
| NDE1 | Mitochondrial NADH dehydrogenase that catalyzes cytosolic NADH oxidation | YMR145c | 28 | 62.7 | 9.3 | X | 81.7 | |
| MDM34 | Protein essential for maintaining wild-type mitochondrial morphology | YGL219c | 5 | 51.9 | 9.1 | 81.3 | ||
| ERG6 | S-Adenosyl-methionine Δ-24-sterol-C-methyltransferase | YML008c | 38 | 43.4 | 5.5 | 80.0 | ||
| SCM4 | Suppressor of Cdc4 mutation | YGR049w | 17 | 20.1 | 8.8 | 77.3 | ||
| ERG9 | Farnesyl-diphosphate farnesyltransferase | YHR190w | 7 | 51.7 | 5.6 | 77.0 | ||
| CBR1 | Cytochrome b reductase | YIL043c | 28 | 36.2 | 9.3 | 75.0 | ||
| SEL1 | Adaptor for Cdc48p-mediated protein degradation via the ubiquitin-proteasome pathway | YML013w | 13 | 66.7 | 5.3 | 74.0 | ||
| FUN14 | Protein of unknown function | YAL008w | 10 | 22.0 | 10.5 | 74.0 | ||
| ILV2 | Acetolactate synthase, first step in valine and isoleucine biosynthesis pathway | YMR108w | 9 | 74.9 | 8.6 | 71.8 | ||
| SSC1 | Mitochondrial heat-shock protein 70 (HSP70) | YJR045c | 25 | 70.6 | 5.5 | X | 70.0 | |
| NCA2 | Control of mitochondrial synthesis of Atp6p and Atp8p | YPR155c | 27 | 70.8 | 6.4 | 65.0 | X | |
| — | Electron-transferring flavoprotein, β chain | YGR207c | 15 | 28.7 | 8.6 | 63.3 | ||
| UIP4 | Protein of unknown function, weak similarity to Xenopus protein xlgv7 | YPL186c | 17 | 34.2 | 4.4 | 61.5 | ||
| EHT1 | Alcohol acyl transferase | YBR177c | 34 | 51.2 | 7.6 | 61.2 | X | |
| LSP1 | Strong similarity to YGR086c | YPL004c | 36 | 38.0 | 4.6 | X | 60.5 | |
| VPS21 | Small GTP-binding protein | YOR089c | 41 | 23.1 | 5.3 | 60.0 | ||
| DPM1 | Dolichyl-phosphate β-d-mannosyltransferase | YPR183w | 25 | 30.3 | 7.7 | 60.0 | ||
| YPT31 | GTP-binding protein of the Rab family | YER031c | 53 | 24.5 | 5.0 | 58.0 | ||
| HSP10 | Heat-shock protein-chaperonin, mitochondrial | YOR020c | 28 | 11.4 | 9.0 | X | 57.0 | |
| MGM1 | Protein that mediates mitochondrial inheritance and is required for mitochondrial outer membrane fusion, member of dynamin family of GTPases | YOR211c | 22 | 101.5 | 8.1 | 55.5 | ||
| IML2 | Protein with a role in stability of artificial minichromosomes | YJL082w | 16 | 82.5 | 6.2 | 54.0 | ||
| SAM35 | Subunit of the mitochondrial sorting and assembly machinery (SAM complex) | YHR083w | 13 | 37.4 | 6.7 | 53.5 | ||
| PTH2 | Aminoacyl-tRNA hydrolase; homologue to mammalian mitochondrial BIT1 (Bcl-2 inhibitor of transcription) | YBL057c | 43 | 23.1 | 5.3 | 52.0 | ||
| GEM1 | Conserved tail-anchored outer mitochondrial membrane GTPase which regulates mitochondrial morphology | YAL048c | 12 | 75.1 | 5.7 | 51.0 | ||
| — | Hypothetical protein | YPR098c | 9 | 17.7 | 9.7 | 51.0 | X | |
| — | Hypothetical protein, similarity to hypothetical Escherichia coli and Caenorhabditis elegans proteins | YER004w | 44 | 25.1 | 9.3 | 50.0 | X | |
| — | Similarity to Emericella nidulans cysteine synthase | YGR012w | 57 | 42.8 | 8.5 | 50.0 | X | |
| PIL1 | Strong similarity to hypothetical protein YPL004c | YGR086c | 38 | 38.3 | 4.5 | X | 49.0 | X |
| — | Hypothetical protein, similarity to aldehyde dehydrogenase | YMR110c | 33 | 59.9 | 6.3 | 48.0 | X | |
| TOM20 | Mitochondrial outer membrane import receptor subunit, 20 kDa | YGR082w | 36 | 20.3 | 5.6 | X | 46.0 | X |
| ATP5 | F1F0-ATPase complex, OSCP subunit | YDR298c | 47 | 22.8 | 9.6 | 38.5 | ||
| YJU3 | Protein containing an α- or β-hydrolase fold domain, has weak similarity to human monoglyceride lipase | YKL094w | 28 | 35.5 | 8.5 | 38.0 | ||
| MIR1 | Mitochondrial phosphate carrier - member of the mitochondrial carrier family | YJR077c | 32 | 32.8 | 9.4 | 37.0 | ||
| ATP15 | F1F0-ATPase complex, F1 epsilon subunit | YPL271w | 84 | 6.7 | 9.8 | X | 36.3 | |
| RHO1 | GTP-binding protein of the Rho subfamily of Ras-like proteins | YPR165w | 13 | 23.1 | 6.0 | 35.0 | ||
| SEC4 | Secretory vesicle-associated Rab GTPase essential for exocytosis | YFL005w | 48 | 23.5 | 6.6 | 34.0 | ||
| YPT32 | Small GTP-binding protein essential for Golgi function | YGL210w | 42 | 24.5 | 5.3 | 33.0 | ||
| ZEO1 | Mid2p-interacting protein, modulates the PKC1-MPK1 cell integrity pathway | YOL109w | 41 | 12.6 | 5.4 | X | 32.7 | |
| CYC1 | Cytochrome c isoform 1 | YJR048w | 55 | 12.1 | 9.5 | 31.2 | X | |
| — | Hypothetical protein | YGR266w | 7 | 81.1 | 6.4 | 30.0 | ||
| FIS1 | Protein involved in mitochondrial division | YIL065c | 46 | 17.7 | 9.2 | 28.5 | X | |
| YPT7 | GTP-binding protein of the RAB family | YML001w | 28 | 23.0 | 4.9 | 28.5 | ||
| POR2 | Putative mitochondrial porin (voltage-dependent anion channel) | YIL114c | 11 | 31.1 | 9.7 | 28.0 | ||
| — | Hypothetical protein, similarity to D. melanogaster heat-shock protein 67B2 | YOR285w | 45 | 15.4 | 5.9 | 27.5 | ||
| FZO1 | Protein involved in mitochondrial fusion and maintenance of the mitochondrial genome | YBR179c | 32 | 97.7 | 6.6 | 26.5 | ||
| TOM7 | Subunit of the preprotein translocase of the mitochondrial outer membrane (TOM complex) | YNL070w | 27 | 6.9 | 8.3 | 24.0 | ||
| AYR1 | 1-Acyldihydroxyacetone-phosphate reductase | YIL124w | 49 | 32.8 | 9.2 | 20.5 | X | |
| POR1 | Mitochondrial porin (voltage-dependent anion channel) | YNL055c | 64 | 30.4 | 7.7 | X | 19.0 | X |
| GTT1 | Glutathione transferase | YIR038c | 21 | 26.8 | 6.2 | 18.7 | ||
| UGO1 | Outer membrane component of the mitochondrial fusion machinery | YDR470c | 21 | 57.4 | 6.6 | 13.5 | ||
| MRPS17 | Protein of the mitochondrial small ribosomal subunit | YMR188c | 26 | 27.6 | 9.6 | 9.0 | ||
| TSC10 | 3-Ketosphinganine reductase | YBR265w | 15 | 36.0 | 5.9 | |||
| MHR1 | Involved in mitochondrial homologous DNA recombination | YDR296w | 20 | 26.9 | 9.5 | |||
| MSP1 | Intramitochondrial sorting protein | YGR028w | 38 | 40.3 | 5.5 | X | ||
| — | Hypothetical protein, strong similarity to molybdopterin-converting factor homologue YKL027w | YHR003c | 38 | 48.8 | 6.4 | X | ||
| YSC83 | Hypothetical protein, strong similarity to Saccharomyces douglasii YSD83 | YHR017w | 24 | 44.2 | 9.1 | |||
| TOM72 | Protein translocase 72-kDa component of the outer membrane of mitochondria | YHR117w | 41 | 71.8 | 5.8 | X | ||
| — | Hypothetical protein, strong similarity to hypothetical protein YHR199c | YHR198c | 26 | 36.5 | 9.1 | |||
| TIM8 | Mitochondrial intermembrane space protein involved in transport of proteins into the inner membrane | YJR135w-a | 24 | 9.8 | 5.2 | |||
| — | Hypothetical protein, similarity to E. coli molybdopterin-converting factor chlN | YKL027w | 62 | 50.3 | 8.9 | X | ||
| SAC1 | Polyphosphoinositide phosphatase, required for transport of ATP into ER | YKL212w | 30 | 71.1 | 7.3 | X | ||
| MMM1 | Protein required for mitochondrial shape and structure | YLL006w | 5 | 48.6 | 5.5 | |||
| ALO1 | d-Arabinono-1,4-lactone oxidase | YML086c | 37 | 59.5 | 6.4 | X | ||
| SAM37 | Subunit of the mitochondrial sorting and assembly machinery (SAM complex) | YMR060c | 17 | 37.4 | 6.9 | |||
| YIM1 | Mitochondrial inner membrane protease | YMR152w | 30 | 41.6 | 7.6 | |||
| TOM40 | Subunit of the preprotein translocase of the mitochondrial outer membrane (TOM complex) | YMR203w | 14 | 42.0 | 5.3 | X | ||
| TOM22 | Subunit of the preprotein translocase of the mitochondrial outer membrane (TOM complex) | YNL131w | 30 | 16.8 | 4.0 | X | ||
| PET123 | Mitochondrial ribosomal protein, small subunit | YOR158w | 19 | 36.0 | 9.8 | |||
| — | Hypothetical protein | YOR228c | 7 | 34.0 | 10.0 | |||
| UBP16 | Ubiquitin-specific protease | YPL072w | 22 | 56.9 | 9.2 | |||
| TOM5 | Subunit of the preprotein translocase of the mitochondrial outer membrane (TOM complex) | YPR133w-a | 30 | 6.0 | 8.1 | |||
| — | Hypothetical protein | YJL133c-a | 36 | 7.7 | 11.4 | |||
| ERG27 | 3-Keto sterol reductase, ERGosterol biosynthesis | YLR100w | 18 | 39.7 | 8.3 | |||
| — | Conserved hypothetical protein | YDR381c-a | 17 | 12.8 | 9.8 | |||
| MDM10 | Subunit of the mitochondrial SAM complex; required for normal mitochondrial morphology and inheritance | YAL010c | 13 | 56.2 | 6.8 | |||
| PDH1 | Hypothetical protein, similarity to Bacillus subtilis mmgE protein | YPR002w | 18 | 57.6 | 9.2 | |||
| MDV1 | Protein involved in mitochondrial division and mitochondrial fission | YJL112w | 8 | 80.0 | 5.4 | |||
| MMR1 | Phosphorylated protein of the mitochondrial outer membrane | YLR190w | 9 | 54.8 | 7.5 | |||
| SEN2 | tRNA splicing endonuclease, β subunit | YLR105c | 17 | 44.1 | 8.6 | |||
| SAM50 | Subunit of the mitochondrial sorting and assembly machinery (SAM complex) | YNL026w | 13 | 54.5 | 8.7 | |||
| TIM9 | Mitochondrial intermembrane space protein involved in transport of proteins into the inner membrane | YEL020w-a | 29 | 10.2 | 8.4 | |||
| SEN54 | tRNA splicing endonuclease, α subunit | YPL083c | 21 | 54.6 | 9.1 | |||
| SEN34 | tRNA splicing endonuclease, γ subunit | YAR008w | 20 | 31.3 | 7.1 | |||
| NUC1 | Nuclease with both DNase and RNase activity, major nuclease of mitochondria | YJL208c | 11 | 37.2 | 9.0 |
Proteins that were also identified in the MSF or via 2D-BAC/SDS-PAGE are indicated (X). MLR, value for mitochondrial localization of mRNA. The identified peptides are listed in Supplemental Table 7. —, no assigned gene name.
Thirteen of the 112 proteins have not been studied previously, i.e., were encoded by an S. cerevisiae open reading frame (ORF) without gene name. The other 99 proteins were encoded by named genes, although a number of these proteins had been previously localized to other cellular compartments or, in particular, to one of the three internal mitochondrial compartments, intermembrane space, inner membrane, and matrix (see below). In the experiments shown in Supplemental Figure S1, we studied several proteins, which were reported to be located in other cellular compartments in some studies: Erg9 and Erg6 that are involved in sterol synthesis, a member of the Rho family (Rho1), Pho88, and the alcohol acyl transferase Eht1 (Leber et al., 1998; Kumar et al., 2002; Huh et al., 2003). We also included two proteins of unknown function and localization: Yor285w, a conserved protein with high homology to the Drosophila melanogaster heat-shock protein 67B2 (Pauli et al., 1988), and Hfd1 (Ymr110c; see below). Localization in the mitochondrial outer membrane was analyzed by different means. 1) Membrane vesicles of purified mitochondria were generated, and outer and inner membrane vesicles were separated by sucrose density gradient centrifugation. We determined whether the proteins carrying a hemagglutinin (HA)-tag cofractionated with the outer membrane marker Tom40 or with the inner membrane marker Tim23 (Supplemental Figure S1, A–C). Erg9, Erg6, and Pho88 each cofractionated exclusively with the outer membrane. (2) Authentic, i.e., nontagged, Yor285w and Rho1 were synthesized in the presence of [35S]methionine/cysteine in reticulocyte lysate and imported into isolated yeast mitochondria. The import was independent of the presence or absence of a membrane potential Δψ across the inner mitochondrial membrane (Supplemental Figure S1D, lanes 2, 3, 7, and 8), and the imported proteins remained largely accessible to proteinase K added to the mitochondria (Supplemental Figure S1D, lanes 4, 5, 9, and 10). Thus, the proteins showed the two characteristic features of mitochondrial outer membrane proteins (Wiedemann et al., 2005). Localization of Yor285w and Rho1 at mitochondria was also reported in independent studies (Sickmann et al., 2003; Ohlmeier et al., 2004). 3) HA-tagged Hfd1 and Eht1 were accessible to protease added to isolated mitochondria (Supplemental Figure S1E; see below; and Figure 3). Thus, each protein analyzed behaved like a typical outer membrane protein, confirming the findings of the mass spectrometry analysis.
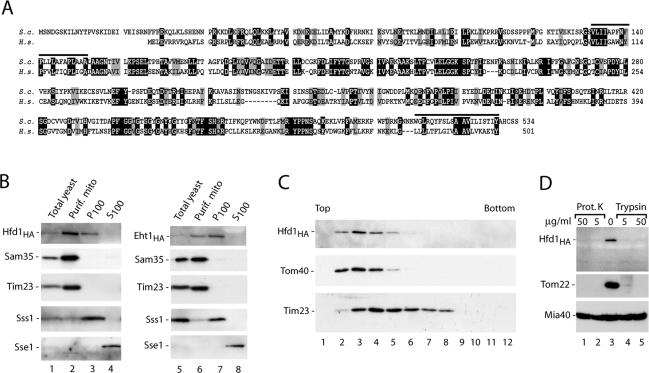
Figure 3.
The homologue of human fatty aldehyde dehydrogenase is mainly located in the mitochondrial outer membrane. (A) Comparison of the predicted primary structures of S. cerevisiae Hfd1, encoded by the open reading frame YMR110c (S.c.), and the human fatty aldehyde dehydrogenase FALDH (H.s.). Black, identical residues; gray, similar residues; bars, predicted hydrophobic transmembrane segments found by the HMMTOP program (Tusnady and Simon, 2001). (B) Subcellular fractionation of yeast strains, containing HA-tagged versions of Hfd1 or Eht1 (Open Biosystems, Brussels, Belgium), according to Meisinger et al. (2000), followed by SDS-PAGE and Western blot analysis. Equal amounts of proteins were loaded. The mitochondrial fraction was purified by sucrose gradient purification. P100, S100, pellet, and supernatant of 100,000 × g centrifugation. Sss1, subunit of the Sec61 protein translocase (endoplasmic reticulum); Sse1, cytosolic heat-shock protein 70. (C) Hfd1HA is located in the outer membrane. Separation of outer and inner membrane vesicles from Hfd1HA mitochondria on a sucrose gradient was performed as described in Materials and Methods. Fractions were analyzed by SDS-PAGE and immunodecoration using antisera against HA, the outer membrane protein Tom40, and the inner membrane protein Tim23. (D) Mitochondrial Hfd1 is accessible to proteases. Highly purified mitochondria (50 μg of protein) isolated from the Hfd1HA strain were resuspended in 100 μl of SEM buffer containing either proteinase K or trypsin and incubated for 15 min on ice. Proteinase K was inhibited by adding 2 mM PMSF, and trypsin was blocked by a 30-fold excess of soybean pancreatic trypsin inhibitor. After washing with SEM, mitochondria were separated on SDS-PAGE and analyzed by Western blotting and immunodecoration.
The Yeast Homologue of Human Fatty Aldehyde Dehydrogenase (FALDH) Is Mainly Located in the Mitochondrial Outer Membrane
The open reading frame YMR110c coded for a new protein of 59.9 kDa with significant similarity to human fatty aldehyde dehydrogenase, including two predicted hydrophobic sequences of sufficient length to function as transmembrane segments (Figure 3A). Mutations in the gene for human FALDH have been shown to be responsible for the Sjögren–Larsson syndrome, an inherited neurocutaneous disorder with mental retardation, spasticity, and ichthyosis (De Laurenzi et al., 1996; Rizzo et al., 2001).
Different views have been reported on the subcellular localization of mammalian fatty aldehyde dehydrogenase, including microsomes, mitochondria, and peroxisomes (Miyauchi et al., 1993; Kelson et al., 1997). Yeast Ymr110c, now termed Hfd1 for homologue of fatty aldehyde dehydrogenase, has been found in so-called lipid particles, intracellular lipid-rich structures consisting of a hydrophobic core with a surrounding phospholipid monolayer and a limited set of proteins (Athenstaedt et al., 1999; Huh et al., 2003). Currently, 37 different yeast proteins have been found in lipid particles (according to the Yeast Proteome Database; www.incyte.com). We identified five of those 37 proteins in the mitochondrial outer membrane proteome: Ayr1, Eht1, Erg6, Faa1, and Hfd1 (Ymr110c). The same five proteins of lipid particles were also found in the proteomic analyses of total yeast mitochondria (Sickmann et al., 2003; Prokisch et al., 2004), whereas most other proteins of lipid particles were not found in mitochondria in any of these proteomic studies. It is thus unlikely that the mitochondrial preparations were simply contaminated with lipid particles. A comparison of the localization data in the literature suggested the possibility of a dual/multiple localization for at least some of these proteins (McCammon et al., 1984; Leber et al., 1998; Kumar et al., 2002; Huh et al., 2003; Sickmann et al., 2003; Prokisch et al., 2004). We performed a cellular fractionation and found HA-tagged Hfd1 in both the purified mitochondrial fraction and the P100 fraction, whereas the mitochondrial marker proteins Sam35 (outer membrane) and Tim23 were only found in the mitochondrial fraction (Figure 3B), supporting the view of a dual localization of Hfd1. For comparison, we determined the localization of HA-tagged Eht1 and found it also both in the mitochondrial and P100 fractions (Figure 3B). Although Eht1 was enriched in the P100 fraction, Hfd1 was mainly located in the highly purified mitochondria (Figure 3B), indicating that mitochondria represented the major cellular location of Hfd1.
The submitochondrial localization of Hfd1 was analyzed by separation of outer and inner membrane vesicles on a sucrose gradient. Hfd1 cofractionated with Tom40 and not with Tim23 (Figure 3C). When isolated mitochondria were treated with proteinase K or trypsin, Hfd1 was degraded like the outer membrane receptor Tom22 (Figure 3D). The mitochondrial intermembrane space protein Mia40 (Chacinska et al., 2004; Naoé et al., 2004; Terziyska et al., 2005) was protected against the proteases, demonstrating that the outer membrane barrier of the mitochondria was not damaged by the protease treatment. Thus, Hfd1HA is exposed on the mitochondrial surface. The localization of Hfd1HA and Eht1HA in the mitochondrial outer membrane was independent of the growth of the cells on nonfermentable medium (Figure 3) or fermentable medium (Supplemental Figure S1, E). Together, these results show that Hfd1 is mainly located in the mitochondrial outer membrane.
Correlation between Preprotein Accumulation at the Mitochondrial Outer Membrane and mRNA Targeting to Mitochondria
Surprisingly, the list of proteins that were identified in the purified outer membrane vesicles (Table 1) contains numerous proteins that are known to be located in one of the three internal mitochondrial compartments. The high purity of the outer membrane vesicles shown in Figure 1C argued against the simple possibility that the outer membranes used here were contaminated with all other mitochondrial compartments. To obtain further evidence, we prepared a mitochondrial surface fraction by a mild treatment with trypsin that did not disrupt the outer membrane barrier (see above; Figure 1D). The peptides identified in the mitochondrial surface fraction belonged to 49 different proteins (Supplemental Table 4). Besides five outer membrane proteins that were accessible to trypsin (like outer membrane TOM-receptors), however, the mitochondrial surface fraction contained mainly proteins known to reside in one of the three internal mitochondrial compartments (Supplemental Table 4). Moreover, several of the proteins from internal mitochondrial compartments were seen as Coomassie-stained spots on the 2D-BAC/SDS-PAGE (Figure 2, Table 1, and Supplemental Table 1), excluding that they were only present in tiny negligible amounts. It should be noted that only one of the 49 proteins identified in the mitochondrial surface fraction had previously been located to a cellular location outside mitochondria, underscoring the purity of the mitochondria used. The nonmitochondrial protein is the large abundant plasma membrane protein Pma2. Pma2 is known to be easily accessible to tryptic digest and mass spectrometric analysis, leading to identification of tiny amounts, and thus represents a frequent contaminant in proteomic analyses (Washburn et al., 2001; Sickmann et al., 2003). We analyzed the distribution of the proteins found in the mitochondrial surface fraction according to their abundance in the cell reported in the yeast database of Ghaemmaghami et al. (2003). The proteins were present in the complete range of abundance without any detectable bias toward proteins of low abundance or high abundance (Supplemental Table 5).
We performed systematic database searches to find a specific characteristic of those proteins that are residents of internal mitochondrial compartments but are also found in outer membrane vesicles and mitochondrial surface fractions. Unexpectedly, we found a striking relation to a genome-wide analysis of mRNAs targeted to yeast mitochondria. Marc et al. (2002) had used yeast DNA microarrays to identify mRNAs found in association with mitochondria. Based on the ratio of mRNA presence in mitochondria-bound polysomes versus free cytosolic polysomes, they defined a value of mitochondrial localization of mRNA (MLR) from 1 (no mitochondrial association) to 100 (mitochondrial association). Marc et al. (2002) compared two populations of mitochondrial proteins: conserved proteins with prokaryotic homologues and proteins that are only found in eukaryotes. The majority of prokaryote-derived mitochondrial proteins possessed MLR values above 70, whereas the majority of eukaryote-derived mitochondrial proteins displayed MLR values below 55. Marc et al. (2002) listed a group of 25 proteins with the highest MLR values of all mitochondrial proteins analyzed. Although only one of those 25 MLR-top scorers was a resident outer membrane protein (Mcr1, which is located both in the outer membrane and the intermembrane space; Hahne et al., 1994), 17 of these 25 proteins were found in the outer membrane/mitochondrial surface fraction.
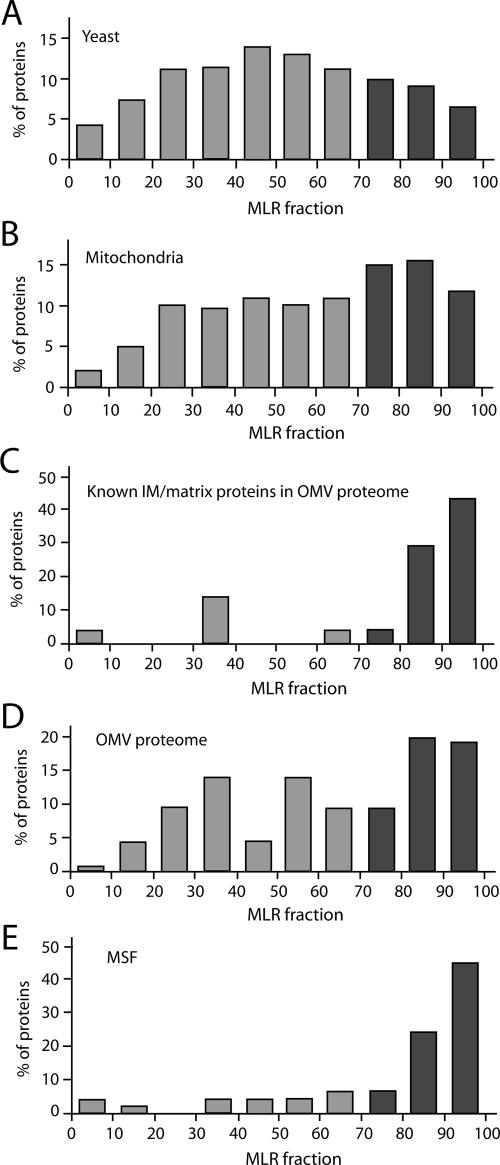
We thus performed a systematic comparison of the MLR values for different populations of yeast proteins. When all yeast proteins analyzed in the genome-wide MLR study are used, a broad distribution of MLR values with a peak at MLR values of 40–60 is observed (Figure 4A) (Marc et al., 2002). By analyzing the proteins found in the yeast mitochondrial proteome by Sickmann et al. (2003), a shift to higher MLR values was observed with a peak at 70–90 (mainly prokaryote-derived mitochondrial proteins), although numerous mitochondrial proteins with low MLR values were also observed (eukaryote derived) (Figure 4B) (Marc et al., 2002). When all known inner membrane and matrix proteins found in the outer membrane proteome were analyzed, most of them displayed MLR values above 80 (Figure 4C and Table 1). This highly asymmetric MLR distribution is significantly different from that of the total outer membrane proteome (Figure 4D). Similarly, the tryptic peptides found in the mitochondrial surface fraction were mostly derived from proteins with MLR values above 80 (Figure 4E and Supplemental Table 4). Thus, proteins with MLR values above 80 found at the mitochondrial outer membrane/surface are highly enriched in prokaryote-derived mitochondrial proteins that are residents of internal mitochondrial compartments.
Figure 4.
Comparison of the mitochondrial outer membrane proteome to a genome-wide analysis of mRNAs targeted to mitochondria. MLR values of 70 or higher (dark columns) indicate a high probability of mRNA localization to the mitochondrial environment (values are accessible at www.biologie.ens.fr/lgmgml/publication/mitoloc/) (Marc et al., 2002). Shown are the MLR fractions (MLR values grouped in blocks of 10: ≥90, ≥80, ≥70, and so on) calculated for all proteins of the classes below for which MLR values are available. (A) Proteins predicted from the S. cerevisiae genome (n = 3190). (B) Mitochondrial proteome (n = 422; Sickmann et al., 2003). (C) Known inner membrane (IM) and matrix proteins found in the outer membrane proteome (n = 21; Table 1). (D) Proteome of outer membrane vesicles (OMV) (n = 79; Table 1). (E) MSF (n = 47; Supplemental Table 4).
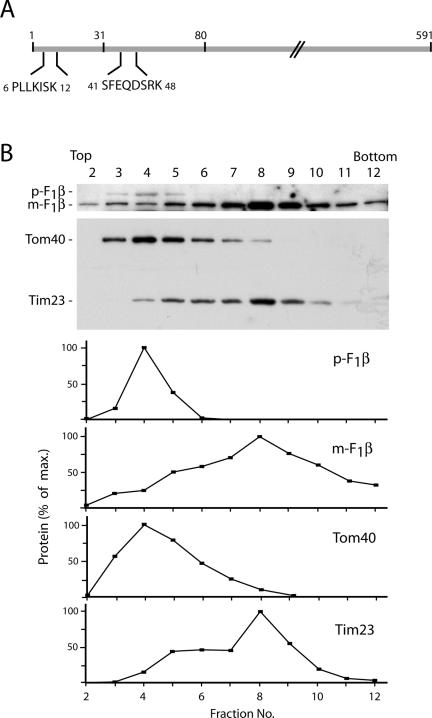
We wondered whether these results based on mRNA targeting could be correlated with results on the protein level. A possible hint was the identification of presequence segments in tryptic peptides analyzed in the proteome of yeast mitochondria (Sickmann et al., 2003). We asked whether the outer membrane vesicles contained presequence forms of mitochondrial proteins. Indeed, the intermembrane space protein cytochrome b2, which was detected as Coomassie-stained protein in the 2D-BAC/SDS-PAGE (Figure 2 and Supplemental Table 1), yielded peptides from its presequence (Figure 5A). Cytochrome b2 is processed in two steps and thus contains two different kinds of presequence segments, an amino-terminal matrix targeting signal and a subsequent inner membrane-sorting signal (Hartl et al., 1987; Glick et al., 1992; Schwarz et al., 1993; Gärtner et al., 1995). An internal peptide of each of these presequence segments was identified (Figure 5A), indicating that the intact precursor form was accumulated in outer membrane vesicles. We used antibodies specific for F0F1-ATPase subunit β (F1β), which is associated with the matrix side of the inner membrane, to study a possible accumulation of the precursor form in outer membrane vesicles. By sonication of mitochondria, we generated outer and inner membrane vesicles and separated them by sucrose density gradient centrifugation. Mature F1β fractionated with the inner membrane protein Tim23 as expected (Figure 5B). In addition, we observed the precursor form of F1β in the Western blot analysis. The precursor protein cofractionated with the outer membrane protein Tom40, demonstrating that the precursor was indeed accumulated at the outer membrane.
Figure 5.
Accumulation of the precursor form of a cleavable mitochondrial protein in the mitochondrial outer membrane. (A) Two presequence peptides of cytochrome b2 identified from the 2D-BAC/SDS-PAGE of purified outer membranes vesicles (Figure 2). Numbers indicate amino acid residues. The processing of cytochrome b2 occurs in two steps, after residue 31 and after residue 80. (B) The precursor form of F1β migrates in the outer membrane fraction. Outer and inner membrane vesicles from wild-type yeast mitochondria were separated on a sucrose gradient, and fractions were analyzed by SDS-PAGE and Western blotting. Immunoreactive bands were quantified and the maximal value was set to 100% for each protein. p-F1β, precursor form; m-F1β, mature protein.
We subjected the 13 new proteins found in the outer membrane proteome to three different prediction programs for amino-terminal presequences, which direct proteins to internal mitochondrial compartments. Three of the proteins, Yjl133c-a, Yhr198c, and Ygr207c, were predicted to contain a characteristic mitochondrial presequence in all three programs (Supplemental Table 6; Nakai and Horton, 1999; Guda et al., 2004; Small et al., 2004), indicating that they belong to the group of internal proteins accumulated as precursors at the outer membrane. For six of the remaining 10 new proteins, MLR values are available (Table 1) (Marc et al., 2002). Each of these MLR values is below 55, suggesting that these proteins without predicted presequence belong to the eukaryote-derived mitochondrial proteins and are mostly resident outer membrane proteins. Indeed, the biochemical analysis of two of these proteins, Hfd1 and Yor285w, confirmed their predominant localization in the mitochondrial outer membrane (see above).
The experiments showing an accumulation of preproteins destined for internal mitochondrial compartments at the outer membrane were performed upon cell growth on nonfermentable medium, i.e., under conditions of high mitochondrial activity and high synthesis rates for mitochondrial proteins. A possible hypothesis for the accumulation of preproteins at the outer membrane would be an overflow of the presequence import pathway that is mainly used by the conserved class of proteins. To test this hypothesis, we prepared mitochondrial surface fractions by treating isolated mitochondria with a low concentration of trypsin upon cell growth at two different conditions, nonfermentable medium and fermentable medium. Silver staining of SDS-gels revealed significant differences in the intensity of a number of bands containing proteins/protein fragments (Supplemental Figure S2A). In most cases, the stained bands were stronger when mitochondria were prepared after cell growth on nonfermentable medium. Using nano-LC-MS/MS, we identified 16 different proteins from internal mitochondrial compartments in these bands. Remarkably, 15 of these proteins possessed high MLR values (Supplemental Figure S2A), indicating a specific enrichment of this subclass of proteins at the outer membrane under nonfermentable conditions. We controlled this finding by Western blot analysis for the matrix protein Hsp60. The isolated mitochondria contained similar amounts of Hsp60 upon growth under nonfermentable and fermentable conditions, but the mitochondrial surface fraction contained a strongly increased level of Hsp60 at nonfermentable conditions (Supplemental Figure S2B), confirming the result obtained by mass spectrometry. Resident proteins of the outer membrane, such as Sam35 and Tom70, were present in similar amounts at both growth conditions in mitochondria as well as in the mitochondrial surface fraction (trypsin-generated fragments of Sam35 and Tom70) (Supplemental Figure S2B). Thus, growth conditions that require a high activity of mitochondria lead to an accumulation of proteins, which possess high MLR values and are residents of internal mitochondrial compartments, at the mitochondrial surface.
DISCUSSION
We have performed a comprehensive analysis of the proteome of mitochondrial outer membranes by a multidimensional approach and obtained a coverage of ∼85% of all known outer membrane proteins. This represents the highest coverage obtained for a proteome of any mitochondrial compartment to date. The approach led to the identification of numerous proteins of low abundance, indicating that the combination of sensitive detection methods with the strong enrichment of proteins in a highly pure submitochondrial fraction yields high sensitivity.
The database reported here represents a rich source for the analysis of new functions of the mitochondrial outer membrane. Interesting examples include a subclass of proteins that are also found in lipid particles and likely possess a dual localization in the cell. A detailed analysis of a new protein (yeast open reading frame YMR110c) previously located to lipid particles revealed a dual localization with a preferential presence in the mitochondrial outer membrane. We termed this protein Hfd1 because it shows significant homology to human fatty aldehyde dehydrogenase that has been shown to be responsible for the inherited Sjögren–Larsson syndrome (De Laurenzi et al., 1996; Rizzo et al., 2001).
The outer membrane proteome includes 12 GTP-binding proteins (Table 1), suggesting that the mitochondrial outer membrane harbors numerous regulatory processes. Many of these proteins belong to the Ras superfamily. In the case of Ypt7, the mammalian homologue Rab32 was found to be specifically associated with mitochondria (Alto et al., 2002). For Gem1, a member of the Rho family, an outer membrane localization and a regulatory function in mitochondrial morphology was recently reported (Frederick et al., 2004). We showed for Rho1, another member of the Rho family identified in the outer membrane proteome, that the in vitro-synthesized protein specifically associates with mitochondria. Another interesting group is formed by proteins involved in ubiquitin-dependent protein degradation, including a ubiquitin-regulatory protein (Sel1) and the ubiquitin-specific protease Ubp16, which was recently found to reside in the mitochondrial outer membrane (Kinner and Kölling, 2003). Two of the new proteins, Ykl027w and Yhr003c, contain motifs that are found in ubiquitin-activating enzymes. We also identified homologues of mammalian proteins involved in apoptosis, such as Pth2 (a homologue of human Bit1; Jan et al., 2004), Ndi1 (a homologue of AMID; Wu et al., 2002), and Yer004w, which is homologous to mammalian HTATIP2, a protein acting in tumor suppression and apoptosis (Hodges et al., 2002). Moreover, we identified three subunits of the tRNA splicing endonuclease complex (Sen2, Sen34, and Sen54) in the purified outer membrane. Indeed, it was recently shown that this complex functions outside of the nucleus in maturation of pre-tRNA (Takano et al., 2005) and shows a preferential location at mitochondria (Yoshihisa et al., 2003). To confirm the results of the mass spectrometry analysis, we determined the cellular localization of several identified proteins, in particular proteins that were previously localized to other cellular compartments and some new proteins. In each case, we found that the proteins were indeed located at the mitochondrial surface. Our findings are in agreement with the view that a significant number of mitochondrial proteins show a dual localization in the cell, i.e., are located in mitochondria and an additional cellular compartment (Mueller et al., 2004; Jeffery, 2005; Karniely and Pines, 2005).
The most surprising observation was that a subclass of proteins, which are known to be residents of internal mitochondrial compartments, i.e., matrix, inner membrane and intermembrane space, were found in the purified outer membranes and in a mitochondrial surface fraction generated by a mild protease treatment of mitochondria. Proteins of low and high abundance were about equally present in this subclass. An integrated analysis, combining protein localization (this study) and mRNA targeting-data (Marc et al., 2002), revealed that most of these proteins were characterized by a high score in a genome-wide analysis for mRNA targeting to mitochondria. mRNA targeting to mitochondria depends on the 3′ untranslated region and involves a mechanism conserved from yeast to human (Margeot et al., 2002; Sylvestre et al., 2003). So far, however, mRNA targeting has received little attention in the protein import field because mitochondrial protein import has been generally assumed to occur via posttranslational protein targeting without a specific function for mRNA targeting (Neupert, 1997; Truscott et al., 2003). The systematic enrichment of precursor proteins with a high mRNA targeting value MLR in the outer membrane proteome provides a first and extensive demonstration of the significance of mRNA targeting on the protein level. This conclusion was independently confirmed by a gradient separation of mitochondrial membrane vesicles, directly demonstrating the presence of the precursor form of a matrix-targeted protein in the outer membrane.
According to the endosymbiont hypothesis, mitochondrial proteins are derived from two different sources, the prokaryotic ancestor of mitochondria and the eukaryotic host cell (Karlberg et al., 2000; Marcotte et al., 2000). Marc et al. (2002) demonstrated that the prokaryote-derived and the eukaryote-derived mitochondrial proteins are significantly different in their MLR values such that the prokaryote-derived proteins typically possess high MLR values, i.e., their mRNAs are targeted to mitochondria (Karlberg and Andersson, 2003). Proteins in the outer membrane proteome and the mitochondrial surface fraction with high MLR values were highly enriched in this conserved subclass of proteins that are residents of internal mitochondrial compartments. The majority of resident outer membrane proteins do not possess prokaryotic homologues but are derived from the eukaryotic host cell (Pfanner et al., 2004), and indeed those proteins mostly display MLR values of low or middle magnitudes. These findings also shed new light on the ongoing discussion of a possible coupling of translation to protein translocation into mitochondria (Kellems et al., 1975; Verner, 1993; George et al., 2002). Indeed, the proteins fumarase (Fum1) and malate dehydrogenase (Mdh1), for which an experimental relation of protein synthesis to protein translocation has been reported (Knox et al., 1998; Fünfschilling and Rospert, 1999; Karniely and Pines, 2005), possess high MLR values and are found in the outer membrane proteome/mitochondrial surface fraction.
The accumulation of the conserved subclass of proteins at the mitochondrial surface was significantly enhanced under nonfermentable growth conditions that require high mitochondrial activity. Thus, large amounts of mitochondrial precursor proteins are synthesized. Because the TIM23 presequence translocase of the inner membrane is four-times less abundant than the outer membrane TOM complex (Dekker et al., 1997), the presequence pathway into the matrix may be overloaded under these conditions, leading to an accumulation of preproteins. Moreover, many proteins of this conserved subclass of proteins are subunits of large mitochondrial complexes such as the respiratory chain complexes that also contain subunits encoded by mitochondrial DNA. Thus, assembly of these complexes depends on the concomitant availability of numerous proteins synthesized in two different locations (Margeot et al., 2005). Under nonfermentable conditions, the rapid mitochondrial growth requires efficient assembly of respiratory chain complexes. Accumulation of the conserved subclass of proteins at the outer membrane under steady-state conditions will ensure a continuous supply (pool) of these proteins to minimize the problem that the conserved nuclear-encoded proteins would be limiting in mitochondrial assembly processes.
In summary, the combination of high-purity cellular subfractionation, comprehensive proteomic analysis, and integrative analysis of protein localization with mRNA targeting and evolutionary relationships provides important new insights into mitochondrial dynamics and biogenesis. The mitochondrial outer membrane is not simply a static membrane that only contains resident proteins; it also accumulates a conserved subclass of mitochondrial precursor proteins.
Supplementary Material
Acknowledgments
We are grateful to Drs. S. Rospert, T. Sommer, W. Kunau, E. Hurt, and T. H. Stevens for antisera and to Drs. C. Jacq and P. Rehling for discussion. We thank I. Perschil for expert technical assistance. This work was supported by the Deutsche Forschungsgemeinschaft (Me 1921/1-1,2, Si 835/2-1,2), FZT-82 (DFG-Forschungszentrum), Sonderforschungsbereich 388, Gottfried Wilhelm Leibniz Program, Max Planck Research Award, Alexander von Humboldt Foundation, Bundesministerium für Bildung und Forschung, and the Fonds der Chemischen Industrie.
This article was published online ahead of print in MBC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E05–08–0740) on January 11, 2005.
Abbreviations used: ACN, acetonitrile; BAC, benzyldimethyl-n-hexadecylammonium chloride; FA, formic acid; F1β, F0F1-ATPase subunit β; HA, hemagglutinin; MALDI, matrix-assisted laser desorption ionization; MLR, mitochondrial localization of mRNA; MS/MS, tandem mass spectrometry; SAM, sorting and assembly machinery; SCX, strong cation exchange; TIM, translocase of inner mitochondrial membrane; TOF, time of flight; TOM, translocase of outer mitochondrial membrane.
The online version of this article contains supplemental material at MBC Online (http://www.molbiolcell.org).
References
- Alconada, A., Gärtner, F., Hönlinger, A., Kübrich, M., and Pfanner, N. (1995). Mitochondrial receptor complex from Neurospora crassa and Saccharomyces cerevisiae. Methods Enzymol. 260, 263–286. [DOI] [PubMed] [Google Scholar]
- Alto, N. M., Soderling, J., and Scott, J. D. (2002). Rab32 is an A-kinase anchoring protein and participates in mitochondrial dynamics. J. Cell Biol. 158, 659–668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Athenstaedt, K., Zweytick, D., Jandrositz, A., Kohlwein, S. D., and Daum, G. (1999). Identification and characterization of major lipid particle proteins of the yeast Saccharomyces cerevisiae. J. Bacteriol. 181, 6441–6448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chacinska, A., Pfannschmidt, S., Wiedemann, N., Kozjak, V., Sanjuán Szklarz, L. K., Schulze-Specking, A., Truscott, K. N., Guiard, B., Meisinger, C., and Pfanner, N. (2004). Essential role of Mia40 in import and assembly of mitochondrial intermembrane space proteins. EMBO J. 23, 3735–3746. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherry, J. M., et al. (1997). Genetic and physical maps of Saccharomyces cerevisiae. Nature 387, 67–73. [PMC free article] [PubMed] [Google Scholar]
- Da Cruz, S., Xenarios, I., Langridge, J., Vilbois, F., Parone, P. A., and Martinou, J. C. (2003). Proteomic analysis of the mouse liver mitochondrial inner membrane. J. Biol. Chem. 278, 41566–41571. [DOI] [PubMed] [Google Scholar]
- Daum, G., Böhni, P. C., and Schatz, G. (1982). Import of preproteins into mitochondria. Cytochrome b2 and cytochrome c peroxidase are located in the intermembrane space of yeast mitochondria. J. Biol. Chem. 257, 13028–13033. [PubMed] [Google Scholar]
- De Laurenzi, V., Rogers, G. R., Hamrock, D. J., Marekov, L. N., Steinert, P. M., Compton, J. G., Markova, N., and Rizzo, W. B. (1996). Sjögren-Larsson syndrome is caused by mutations in the fatty aldehyde dehydrogenase gene. Nat. Genet. 12, 52–57. [DOI] [PubMed] [Google Scholar]
- Dekker, P. J., Martin, F., Maarse, A. C., Bömer, U., Müller, H., Guiard, B., Meijer, M., Rassow, J., and Pfanner, N. (1997). The Tim core complex defines the number of mitochondrial contact sites and can hold arrested preproteins in the absence of matrix Hsp70-Tim44. EMBO J. 16, 5408–5419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Endo, T., Yamamoto, H., and Esaki, M. (2003). Functional cooperation and separation of translocators in protein import into mitochondria, the double-membrane bounded organelles. J. Cell Sci. 116, 3259–3267. [DOI] [PubMed] [Google Scholar]
- Frederick, R. L., McCaffery, J. M., Cunningham, K. W., Okamoto, K., and Shaw, J. M. (2004). Yeast Miro GTPase, Gem1, regulates mitochondrial morphology via a novel pathway. J. Cell Biol. 167, 87–98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fünfschilling, U., and Rospert, S. (1999). Nascent polypeptide-associated complex stimulates protein import into yeast mitochondria. Mol. Biol. Cell 10, 3289–3299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gärtner, F., Bömer, U., Guiard, B., and Pfanner, N. (1995). The sorting signal of cytochrome b2 promotes early divergence from the general mitochondrial import pathway and restricts the unfoldase activity of matrix Hsp70. EMBO J. 14, 6043–6057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- George, R., Walsh, P., Beddoe, T., and Lithgow, T. (2002). The nascent polypeptide-associated complex (NAC) promotes interaction of ribosomes with the mitochondrial surface in vivo. FEBS Lett. 516, 213–216. [DOI] [PubMed] [Google Scholar]
- Ghaemmaghami, S., Huh, W. K., Bower, K., Howson, R. W., Belle, A., Dephoure, N., O'Shea, E. K., and Weissman, J. S. (2003). Global analysis of protein expression in yeast. Nature 425, 737–741. [DOI] [PubMed] [Google Scholar]
- Glick, B. S., Brandt, A., Cunningham, K., Müller, S., Hallberg, R. L., and Schatz, G. (1992). Cytochromes c1 and b2 are sorted to the intermembrane space of yeast mitochondria by a stop-transfer mechanism. Cell 69, 809–822. [DOI] [PubMed] [Google Scholar]
- Green, D. R., and Kroemer, G. (2004). The pathophysiology of mitochondrial cell death. Science 305, 626–629. [DOI] [PubMed] [Google Scholar]
- Guda, C., Guda, P., Fahy, E., and Subramaniam, S. (2004). MITOPRED: a Web server for the prediction of mitochondrial proteins. Nucleic Acids Res. 32, W372–W374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahne, K., Haucke, V., Ramage, L., and Schatz, G. (1994). Incomplete arrest in the outer membrane sorts NADH-cytochrome b5 reductase to two different submitochondrial compartments. Cell 79, 829–839. [DOI] [PubMed] [Google Scholar]
- Hartl, F. U., Ostermann, J., Guiard, B., and Neupert, W. (1987). Successive translocation into and out of the mitochondrial matrix: targeting of proteins to the intermembrane space by a bipartite signal peptide. Cell 51, 1027–1037. [DOI] [PubMed] [Google Scholar]
- Hill, K., Model, K., Ryan, M. T., Dietmeier, K., Martin, F., Wagner, R., and Pfanner, N. (1998). Tom40 forms the hydrophilic channel of the mitochondrial import pore for preproteins. Nature 395, 516–521. [DOI] [PubMed] [Google Scholar]
- Hodges, P. E., Carrico, P. M., Hogan, J. D., O'Neil, K. E., Owen, J. J., Mangan, M., Davis, B. P., Brooks, J. E., and Garrels, J. L. (2002). Annotating the human proteome: the human proteome survey database (humanPSD) and an indepth target database for G-protein-coupled receptors (GPCR-PD) from Incyte Genomics. Nucleic Acids Res. 30, 137–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huh, W. K., Falvo, J. V., Gerke, L. C., Carroll, A. S., Howson, R. W., Weissman, J. S., and O'Shea, E. K. (2003). Global analysis of protein localization in budding yeast. Nature 425, 686–691. [DOI] [PubMed] [Google Scholar]
- Jan, Y., Matter, M., Pai, J., Chen, Y., Pilch, J., Komatsu, M., Ong, E., Fukuda, M., and Ruoshlati, E. (2004). A mitochondrial protein, Bit1, mediates apoptosis regulated by integrins and Groucho/TLE corepressors. Cell 116, 751–762. [DOI] [PubMed] [Google Scholar]
- Jensen, R. E., and Dunn, C. D. (2002). Protein import into and across the mitochondrial inner membrane: role of the TIM23 and TIM22 translocons. Biochim. Biophys. Acta 1592, 25–34. [DOI] [PubMed] [Google Scholar]
- Jensen, R. E., Dunn, C. D., Youngman, M. J., and Sesaki, H. (2004). Mitochondrial building blocks. Trends Cell Biol. 14, 215-218. [DOI] [PubMed] [Google Scholar]
- Jeffery, C. J. (2005). Mass spectrometry and the search for moonlighting proteins. Mass Spectrom. Rev. 24, 772–782. [DOI] [PubMed] [Google Scholar]
- Karlberg, E. O., and Andersson, S. G. (2003). Mitochondrial gene history and mRNA localization: is there a correlation? Nat. Rev. Genet. 4, 391–397. [DOI] [PubMed] [Google Scholar]
- Karlberg, O., Canback, B., Kurland, C. G., and Andersson, S. G. (2000). The dual origin of the yeast mitochondrial proteome. Yeast 17, 170–187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karniely, S., and Pines, O. (2005). Single translation–dual destination: mechanisms of dual protein targeting in eukaryotes. EMBO Rep. 6, 420–425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellems, R. E., Allison, V. F., and Butow, R. A. (1975). Cytoplasmic type 80S ribosomes associated with yeast mitochondria. IV. Attachment of ribosomes to the outer membrane of isolated mitochondria. J. Cell Biol. 65, 1–14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelson, T. L., Secor McVoy, J. R., and Rizzo, W. B. (1997). Human liver fatty aldehyde dehydrogenase: microsomal localization, purification, and biochemical characterization. Biochim. Biophys. Acta 1335, 99–110. [DOI] [PubMed] [Google Scholar]
- Kinner, A., and Kölling, R. (2003). The yeast deubiquinating enzyme Ubp16 is anchored to the outer mitochondrial membrane. FEBS Lett. 549, 135–140. [DOI] [PubMed] [Google Scholar]
- Knox, C., Sass, E., Neupert, W., and Pines, O. (1998). Import into mitochondria, folding and retrograde movement of fumarase in yeast. J. Biol. Chem. 273, 25587–25593. [DOI] [PubMed] [Google Scholar]
- Koehler, C. M. (2004). New developments in mitochondrial assembly. Annu. Rev. Cell Dev. Biol. 20, 309–335. [DOI] [PubMed] [Google Scholar]
- Kumar, A., et al. (2002). Subcellular localization of the yeast proteome. Genes Dev. 16, 707–719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leber, R., Landl, K., Zinser, E., Ahorn, H., Spok, A., Kohlwein, S. D., Turnowsky, F., and Daum, G. (1998). Dual localization of squalene epoxidase, Erg1p, in yeast reflects a relationship between the endoplasmic reticulum and lipid particles. Mol. Biol. Cell 9, 375–386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lill, R., and Mühlenhoff, U. (2005). Iron-sulfur-protein biogenesis in eukaryotes. Trends Biochem. Sci. 30, 133–141. [DOI] [PubMed] [Google Scholar]
- Marcotte, E. M., Xenarios, I., van Der Bliek, A. M., and Eisenberg, D. (2000). Localizing proteins in the cell from their phylogenetic profiles. Proc. Natl. Acad. Sci. USA 97, 12115–12120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc, P., Margeot, A., Devaux, F., Blugeon, C., Corral-Debrinski, M., and Jacq, C. (2002). Genome-wide analysis of mRNAs targeted to yeast mitochondria. EMBO Rep. 3, 159–164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeot, A., Blugeon, C., Sylvestre, J., Vialette, S., Jacq, C., and Corral-Debrinski, M. (2002). In Saccharomyces cerevisiae, ATP2 mRNA sorting to the vicinity of mitochondria is essential for respiratory function. EMBO J. 21, 6893–6904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margeot, A., Garcia, M., Wang, W., Tetaud, E., di Rago, J. P., and Jacq, C. (2005). Why are so many mRNAs translated to the vicinity of mitochondria: a role in protein complex assembly? Gene 354, 64–71. [DOI] [PubMed] [Google Scholar]
- McCammon, M. T., Hartmann, M. A., Bottema, C. D., and Parks, L. W. (1984). Sterol methylation in Saccharomyces cerevisiae. J. Bacteriol. 157, 475–483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger, C., Ryan, M. T., Hill, K., Model, K., Lim, J. H., Sickmann, A., Müller, H., Meyer, H. E., Wagner, R., and Pfanner, N. (2001). Protein import channel of the outer mitochondrial membrane: a highly stable Tom40-Tom22 core structure differentially interacts with preproteins, small Tom proteins, and import receptors. Mol. Cell Biol. 21, 2337–2348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meisinger, C., Sommer, T., and Pfanner, N. (2000). Purification of Saccharomyces cerevisiae mitochondria devoid of microsomal and cytosolic contaminations. Anal. Biochem. 287, 339–342. [DOI] [PubMed] [Google Scholar]
- Mihara, K. (2003). Cell biology: moving inside membranes. Nature 424, 505–506. [DOI] [PubMed] [Google Scholar]
- Mitulovic, G., et al. (2003). An improved method for tracking and reducing the void volume in nano HPLC-MS with micro trapping columns. Anal. Bioanal. Chem. 376, 946–951. [DOI] [PubMed] [Google Scholar]
- Mitulovic, G., et al. (2004). Automated, on-line two-dimensional nano liquid chromatography tandem mass spectrometry for rapid analysis of complex protein digests. Proteomics 4, 2545–2557. [DOI] [PubMed] [Google Scholar]
- Miyauchi, K., Yamamoto, A., Masaki, R., Fujiki, Y., and Tashiro, Y. (1993). Microsomal aldehyde dehydrogenase or its cross-reacting protein exists in outer mitochondrial membranes and peroxisomal membranes in rat liver. Cell Struct. Funct. 18, 427–436. [DOI] [PubMed] [Google Scholar]
- Mootha, V. K., et al. (2003). Integrated analysis of protein composition, tissue diversity, and gene regulation in mouse mitochondria. Cell 115, 629–640. [DOI] [PubMed] [Google Scholar]
- Mueller, J. C., Andreoli, C., Prokisch, H., and Meitinger, T. (2004). Mechanisms for multiple intracellular localization of human mitochondrial proteins. Mitochondrion 3, 315–325. [DOI] [PubMed] [Google Scholar]
- Nakai, K., and Horton, P. (1999). PSORT: a program for detecting the sorting signals of proteins and predicting their subcellular localization Trends Biochem. Sci. 24, 34–35. [DOI] [PubMed] [Google Scholar]
- Naoé, M., Ohwa, Y., Ishikawa, D., Ohshima, C., Nishikawa, S., Yamamoto, H., and Endo, T. (2004). Identification of Tim40 that mediates protein sorting to the mitochondrial intermembrane space. J. Biol. Chem. 279, 47815–47821. [DOI] [PubMed] [Google Scholar]
- Neupert, W. (1997). Protein import into mitochondria. Annu. Rev. Biochem. 66, 863–917. [DOI] [PubMed] [Google Scholar]
- Newmeyer, D. D., and Ferguson-Miller, S. (2003). Mitochondria: releasing power for life and unleashing the machine of death. Cell 112, 481–490. [DOI] [PubMed] [Google Scholar]
- Ohlmeier, S., Kastaniotis, A. J., Hiltunen, J. K., and Bergmann, U. (2004). The yeast mitochondrial proteome, a study of fermentative and respiratory growth. J. Biol. Chem. 279, 3956–3979. [DOI] [PubMed] [Google Scholar]
- Pauli, D., Tonka, C. H., and Ayme-Southgate, A. (1988). An unusual split Drosophila heat shock gene expressed during embryogenesis, pupation and in testis. J. Mol. Biol. 200, 47–53. [DOI] [PubMed] [Google Scholar]
- Pfanner, N., Wiedemann, N., Meisinger, C., and Lithgow, T. (2004). Assembling the mitochondrial outer membrane. Nat. Struct. Mol. Biol. 11, 1044–1048. [DOI] [PubMed] [Google Scholar]
- Prokisch, H., et al. (2004). Integrative analysis of the mitochondrial proteome in yeast. PLoS Biol. 2, e160. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reichert, A. S., and Neupert, W. (2004). Mitochondriomics or what makes us breathe. Trends Genet. 20, 555–562. [DOI] [PubMed] [Google Scholar]
- Rizzo, W. B., Lin, Z., and Carney, G. (2001). Fatty aldehyde dehydrogenase: genomic structure, expression and mutation analysis in Sjögren-Larsson syndrome. Chem. Biol. Interact. 130–132, 297–307. [DOI] [PubMed] [Google Scholar]
- Schapira, A. H. (2000). Mitochondrial disorders. Curr. Opin. Neurol. 13, 527–532. [DOI] [PubMed] [Google Scholar]
- Scheffler, I. E. (1999). Mitochondria, New York: Wiley.
- Schon, E. A. (2000). Mitochondrial genetics and disease. Trends Biochem. Sci. 25, 555–560. [DOI] [PubMed] [Google Scholar]
- Schwarz, E., Seytter, T., Guiard, B., and Neupert, W. (1993). Targeting of cytochrome b2 into the mitochondrial intermembrane space: specific recognition of the sorting signal. EMBO J. 12, 2295–2302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shevchenko, A., Wilm, M., Vorm, O., and Mann, M. (1996). Mass spectrometric sequencing of proteins silver-stained polyacrylamide gels. Anal. Chem. 68, 850–858. [DOI] [PubMed] [Google Scholar]
- Sickmann, A., et al. (2003). The proteome of Saccharomyces cerevisiae mitochondria. Proc. Natl. Acad. Sci. USA 100, 13207–13212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sikorski, R. S., and Hieter, P. (1989). A system of shuttle vectors and yeast host strains designed for efficient manipulation of DNA in Saccharomyces cerevisiae. Genetics 122, 19–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog, B., and Wichmann, A. (1986). Calculation of the isoelectric points of poly-peptides from the amino acid composition. Trends Analyt. Chem. 5, 82–83. [Google Scholar]
- Small, I., Peeters, N., Legeai, F., and Lurin, C. (2004). Predotar: a tool for rapidly screening proteomes for N-terminal targeting sequences. Proteomics 4, 1581–1590. [DOI] [PubMed] [Google Scholar]
- Steinmetz, L. M., et al. (2002). Systematic screen for human disease genes in yeast. Nat. Genet. 31, 400–404. [DOI] [PubMed] [Google Scholar]
- Sylvestre, J., Margeot, A., Jacq, C., Dujardin, G., and Corral-Debrinski, M. (2003). The role of the 3′ untranslated region in mRNA sorting to the vicinity of mitochondria is conserved from yeast to human cells. Mol. Biol. Cell 14, 3848–3856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takano, A., Endo, T., and Yoshihisa, T. (2005). tRNA actively shuttles between the nucleus and cytosol in yeast. Science 309, 140–142. [DOI] [PubMed] [Google Scholar]
- Taylor, S. W., et al. (2003). Characterization of the human heart mitochondrial proteome. Nat. Biotechnol. 21, 281–286. [DOI] [PubMed] [Google Scholar]
- Terziyska, N., Lutz, T., Kozany, C., Mokranjac, D., Mesecke, N., Neupert, W., Herrmann, J. M., and Hell, K. (2005). Mia40, a novel factor for protein import into the intermembrane space of mitochondria is able to bind metal ions. FEBS Lett. 579, 179–184. [DOI] [PubMed] [Google Scholar]
- Truscott, K. N., Brandner, K., and Pfanner, N. (2003). Mechanisms of protein import into mitochondria. Curr. Biol. 13, R326–R337. [DOI] [PubMed] [Google Scholar]
- Tusnady, G. E., and Simon, I. (2001). The HMMTOP transmembrane topology prediction server. Bioinformatics 17, 849–850. [DOI] [PubMed] [Google Scholar]
- van Wilpe, S., et al. (1999). Tom22 is a multifunctional organizer of the mitochondrial preprotein translocase. Nature 401, 485–489. [DOI] [PubMed] [Google Scholar]
- Verner, K. (1993). Co-translational protein import into mitochondria: an alternative view. Trends Biochem. Sci. 18, 366–371. [DOI] [PubMed] [Google Scholar]
- Wagner, Y., Sickmann, A., Meyer, H. E., and Daum, G. (2003). Multidimensional nano-HPLC for analysis of protein complexes. J. Am. Soc. Mass. Spectrom. 14, 1003–1011. [DOI] [PubMed] [Google Scholar]
- Wallace, D. C. (2005). A mitochondrial paradigm of metabolic and degenerative diseases, aging, and cancer: a dawn for evolutionary medicine. Annu. Rev. Genet. 39, 359–407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washburn, M. P., Wolters, D., and Yates, J. R. (2001). Large-scale analysis of the yeast proteome by multidimensional protein identification technology. Nat. Biotechnol. 19, 242–247. [DOI] [PubMed] [Google Scholar]
- Wiedemann, N., Kozjak, V., Chacinska, A., Schönfisch, B., Rospert, S., Ryan, M. T., Pfanner, N., and Meisinger, C. (2003). Machinery for protein sorting and assembly in the mitochondrial outer membrane. Nature 424, 565–571. [DOI] [PubMed] [Google Scholar]
- Wiedemann, N., Pfanner, N., and Rehling, P. (2005). Import of precursor proteins into isolated yeast mitochondria. Methods Mol. Biol. 313, 373–383. [DOI] [PubMed] [Google Scholar]
- Wu, M., Xu, L.G., Li, X., Zhai, Z., and Shu, H. B. (2002). AMID, an apoptosis-inducing factor-homologous mitochondrion-associated protein, induces caspase-independent apoptosis. J. Biol. Chem. 277, 25617–25623. [DOI] [PubMed] [Google Scholar]
- Yoshihisa, T., Yunoki-Esaki, K., Ohshima, C., Tanaka, N., and Endo, T. (2003). Possibility of cytoplasmic pre-tRNA splicing: the yeast tRNA splicing endonuclease mainly localizes on the mitochondria. Mol. Biol. Cell 14, 3266–3279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zahedi, R. P., Meisinger, C., and Sickmann, A. (2005). Two-dimensional benzyldimethyl-n-hexadecylammonium chloride/SDS-PAGE for membrane proteomics. Proteomics 5, 3581–3588. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.