Abstract
Background
Previously, we showed that the 27nt repeat polymorphism in endothelial nitric oxide synthase (eNOS) intron 4 was associated with altered eNOS mRNA and protein levels, nitric oxide (NO) production and vascular disease risk; the 27-nt repeats had a cis-acting role in eNOS promoter function. In the present study, we investigated nuclear protein that binds the 27nt repeat and mediates eNOS expression.
Methods and Results
Using 5′-biotin-labeled 27nt DNA duplex and streptavidin–agarose beads pull-down assay and mass spectrometry, we identified that nuclear β-actin was one of the major 27nt binding proteins. Using the pGL3 reporter vectors containing the 5 × 27nt repeats as an enhancer in an in vitro transcription assay, we found that exogenous β-actin significantly increased reporter gene transcription efficiency. The β-actin’s upregulating effect was compromised when exogenous 27nt RNA duplex was added. Furthermore, the eNOS expression was reduced when β-actin gene was silenced by specific siRNA, and actin overexpression upregulated eNOS expression > 3-fold.
Conclusion
Our data demonstrate that β-actin as a transcription factor stimulates eNOS expression; and the transcriptional effect appears to be 27nt-dependent. Our findings represent a novel molecular mechanism regulating eNOS expression, which could potentially lead to discoveries of eNOS specific pharmaceutical agents, eg, active peptides, with clinical applications.
Keywords: endothelial nitric oxide synthase, intron 4, nuclear β-actin
Nitric oxide (NO) plays a vital role in vascular homeostasis by maintaining endothelial functional integrity, regulating vascular tone, suppressing vascular smooth muscle cell proliferation, and inhibiting monocytes, leukocyte adhesion, and platelet aggregation.1 Whereas variation in endothelial nitric oxide synthase (eNOS) enzyme activity has been the main focus on eNOS regulation, alterations in eNOS expression also play an important role in the bioavailability of the eNOS, hence vascular NO.2,3 Previously, we have shown that the 27nt repeat polymorphism in eNOS intron 4 is associated with altered eNOS mRNA and protein levels, NO production, and risk of vascular diseases.4–6 Recently, we have further demonstrated that the 27nt repeats may have a cis-acting role in the eNOS promoter function and transcription efficiency.7 Using electrophoresis mobility shift assay, we have demonstrated that there are nuclear proteins bound with the 27nt DNA repeat fragment.7 However, there are no obvious consensus transcription factor binding sites within the fragment (5′-GAAGTCTAGACCTGCTGCAGGGGTGAG-3). In the present study, we have extended our investigation to identify these binding proteins and examined the functional properties of the endothelial nuclear proteins in the eNOS regulation. Our data show that endothelial nuclear β-actin is one of the binding proteins to the 27nt repeats. This binding appears to upregulate eNOS transcriptional efficiency; its effect depends on the presence of the 27nt repeats as an enhancer. Our findings that nuclear β-actin regulates the eNOS expression in a 27nt repeats-dependent manner represent a novel molecular mechanism for the eNOS gene regulation in human vascular endothelial cells.
Materials and Methods
See online supplemental data (http://atvb.ahajournals.org) for detailed Methods.
siRNA Preparation
We designed the target-specific 19nt (referred as siRNA) RNA duplexes according to sequences of the type AA(N19)UU (N, any nucleotide) from the β-actin mRNA. The 19nt siRNA sequences targeting β-actin (NM_001101) at position 974 to 992 were: 5′-GGCGGCACCACCATGTACC-3′ (sense), 5′-GGTACATGGTGGTGCCGCC-3′ (antisense). We also prepared negative controls using the mRNA sequence of the firefly luciferase gene (X65324) 153 to 171 for the 19nt siRNA: 5′-GUCUGACAGUUACCAAUGC-3′ (sense), 5′-GCAUUGGUAACUGUCAGAC-3′ (antisense). These siRNA sequences were submitted to a BLAST search against the human genome sequences other than the β-actin. All siRNAs were chemically synthesized (Integrated DNA Technologies, Inc., Coralville, Iowa).
In Vitro Transcription Assay
HelaScribe Nuclear Extract in vitro Transcription System and the cloned plasmids of the pGL3-promoter with or without the 27nt repeats of the eNOS intron 4 were used in the reaction. A standard transcription reaction was carried out in a solution of 25 μL containing 3.4 μL endothelial nuclear extract (8.0 U/reaction). The 27nt RNA duplex homologous to the 27nt repeat fragment or β-actin was added to the reaction as indicated. As the control, we also synthesized a 27nt RNA duplex with the sequence homologous to a part of the eNOS exon 24 (5′-GCGACGAGGTGCAGAACGCCCAGCAGC-3′). The purity and quantity of the extracted RNA were tested using spectrometry before subjected to the quantitative real-time reverse-transcription polymerase chain reaction for mRNA measurements of specific genes.
Cell Culture, Treatment, and Transfection
Human aortic endothelial cells (HAECs) (Cell Application, Inc) between passages 4 and 8 were used in all experiments. Cells were grown up to 70% to 80% confluence before transfection. The siRNA duplex was transfected using Lipofectamine 2000 in OptiMem-I media. After 4 hours, the transfection medium was replaced by fresh EBM-2 containing 3% fetal bovine serum. Except for the time-course assays, cells were harvested for RNA or protein extraction at 24 or 48 hours after transfection.
For β-actin overexpression in endothelial cells, we used plasmids containing the human β-actin coding sequence (pAcGFP1-Actin; BD Science Clontech, Palo Alto, Calif), and the control plasmid without the β-actin gene (pAcGFP-C1). All other transfection conditions were the same as that for the siRNA. The transfected endothelial cells were collected at the end of designated experimental periods for the measurements of eNOS expression.
Isolation of Nuclear Extracts and Streptavidin-Bead Precipitation Assay
HAECs were used for nuclear protein extraction; and the 5′-biotin labeled 27nt DNA duplex was used in the pull-down assays. The eluted nuclear proteins were subjected to a 2D gel electrophoresis (Kendrick Laboratory, Inc, Madison, Wis); the spots were visualized using Coomassie blue. The 5 differentiated spots were cut off the Coomassie-stained gel before digested with trypsin. The resulting peptide mixture was analyzed by MALDI-MS; the profiles of the peptide masses obtained by the MALDI-MS were matched with the profile of known proteins in the database (http://www.expasy.ch).
Results
Identification of Nuclear Proteins Binding to the 27nt Duplex
We previously reported that there was a binding between endothelial nuclear protein(s) and 27nt repeats of the intron 4 by electrophoresis mobility shift assay.7 To identify the protein(s) binding to the 27nt repeats, we used streptavidin–agarose bead-based oligonucleotide pull-down technique. The 5′-biotin-labeled 27nt DNA duplex was mixed with endothelial nuclear protein extracts before magnetic force was applied. The proteins eluted from the streptavidin-agarose beads were separated on a 2D electrophoresis. The separated spots were subjected to MALDI-MS analyses, which identified β-actin, nucleophosmin (B23), keratin 9, and annexin-2 as the binding proteins (see Figure I for the 2D gel, available online at http://atvb.ahajournals.org).
We further confirmed the presence of the β-actin (42kD) in the pull-down complex using a specific anti–β-actin IgG (Figure I). Using the commercial β-actin protein preparation (200 ng/μL) replacing the nuclear extracts to react directly with the labeled 27nt DNA duplex, we also showed that the β-actin was specifically bound to the 27nt DNA fragment (Figure I). Yet no β-actin was observed in biotin-labeled 27nt DNA fragment with sequence from a randomly selected segment within the exon 24 of the eNOS gene (D26607, position at 21207 to 21234: 5′-AGCCCACTCCCATGACTTTGGTGTTCG-3′). These data demonstrate that β-actin specifically binds to the 27nt DNA duplex.
β-Actin Exists Within the Nucleus of the Vascular Endothelial Cells
Given the fact that monomeric β-actin is present in nucleus and transcriptionally active in nonendothelial cells,8–11 we next investigated hypothesis that β-actin could potentially regulate eNOS expression via interaction with the 27nt repeats in endothelial cells. We used anti-actin 2G2, which was used to show nuclear actin through specific binding epitope of 2G2 on the actin in the nuclei of myogenic and fibroblastic cells,11 to detect the intracellular distribution of the β-actin in the cultured HAECs. A representative image of the nuclear actin stained by the secondary antibody labeled with Texas Red was shown in Figure 1A. The actin appeared to be clustered in the punctate form in the center of HAEC nucleus. To further confirm this observation, we performed a Western blotting analysis using the fractional extracts of the cytosol and the nuclei. As shown in Figure 1B, the actin recognized by the anti-actin 2G2 was observed in both the cytosolic and nuclear fractions. As expected, the anti-actin 2G2 also recognized the cytoplasm actin. Meanwhile, vimentin was only detectable in the cytosolic but not in the nuclear fraction (Figure 1B), ruling out the possibility of cytosolic contamination in the nuclear extracts. These results have clearly shown that β-actin exists within the nuclei of the endothelial cells.
Figure 1.
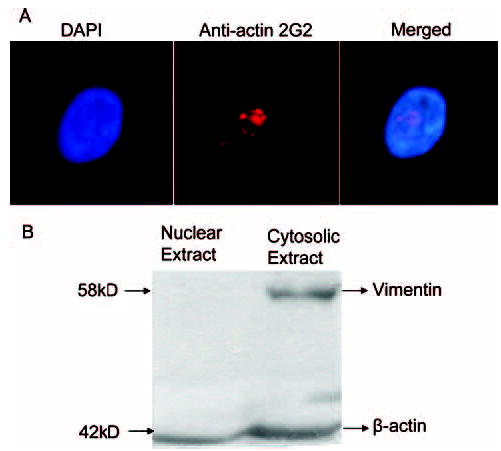
Nuclear localization of the β-actin in cultured endothelial cells A, HAECs were cultured and grown up to 50% confluence. After fixed by methanol and acetone (v/v, 1:1), cells were blocked with blocking buffer and incubated with the mouse anti-2G2 monoclonal antibody, which was detected by the Texas Red labeled goat anti-mouse antibody. The nuclear β-actin was stained red and observed under fluorescence microscopy. The nucleus was shown by the DAPI staining (blue). B, Fractionated cytosolic and nuclear extracts were subjected to Western blotting with anti-vimentin antibody and re-probed with the 2G2 anti–β-actin antibody. Vimentin was only present in the cytosolic fraction, and the 2G2 antibody appeared to recognize both nuclear and cytosolic β-actin.
27nt Repeat Insertion Enhances the Stimulatory Effect of the β-Actin on Reporter Gene Transcription Efficiency
Next, we tested whether the nuclear β-actin is functional in regulating eNOS gene transcription in vitro. Our previous study showed that the 27nt repeats could be potentially functional as an enhancer/repressor in eNOS gene transcription.7 The recombinant pGL3 reporter vector was inserted with 5 × 27nt DNA repeats to replace the SV40 enhancer at the 3′ end of the luciferase gene coding sequence. Plasmids with either SV40 enhancer or no enhancer/repressor were used as controls. Using these recombinant pGL3 plasmids, we added β-actin or anti–β-actin antibody into the in vitro transcription system, which contained endothelial nuclear extracts. The same amount of bovine serum albumin was used as a control for the effects of β-actin or anti–β-actin. We showed that the pGL3 plasmids containing the 5 × 27nt repeats had a significantly higher transcription efficiency (7.25 ± 1.68 × 10−6) than the plasmids containing the SV40 enhancer (4.32 ± 0.92 × 10−6, P < 0.01), or without an enhancer (0.99 ± 0.38 × 10−6, P < 0.01). Addition of β-actin to the reaction mixture resulted in a dramatic increase in the transcription efficiency in plasmids containing the 5 × 27nt (12.76 ± 2.33 × 10−6) or the SV40 enhancer (7.99 ± 2.01 × 10−6), but not in the basic vector (0.73 ± 0.21 × 10−6). We also observed that the stimulatory action of the β-actin on the report gene transcription efficiency was in a dose-dependent manner in the concentration range from 0 to 10 μg/mL (data not shown). However, when anti–β-actin antibody was added into the reaction system, which contained endogenous nuclear actin in the endothelial nuclear extracts, the luciferase gene transcription efficiency was decreased by 55.8% (4.32 ± 0.92 × 10−6 versus 1.91 ± 0.86 × 10−6 P < 0.01) in SV40 enhancer-containing plasmids, and by 58.3% (7.25 ± 1.68 × 10−6 versus 3.02 ± 1.20 × 10−6, P < 0.01) in the plasmids containing the 5 × 27nt repeats as an enhancer. This finding suggests that β-actin may upregulate transcription efficiency in genes containing an enhancer. The upregulating effect does not appear to be confined to the 27nt repeats only. It is also effective on the SV40 enhancer. The data further support our previous finding that the 27nt repeats may function as an intrinsic enhancer for the eNOS transcription.7
Exogenous 27nt RNA Duplex Interrupts the Stimulatory Effect of the β-Actin on the Transcription Efficiency
We further studied the specificity for the interaction between β-actin and the 27nt repeats on the transcription efficiency. In the reaction mixture of the in vitro transcription assay, we used the 2 plasmids presented above with either the 5 × 27nt repeats or the SV40 enhancer for the luciferase gene. The chemically synthesized 27nt RNA duplex, which is homologous to the sequence of 27nt repeats of the intron 4, was used to test for the effects on actin-induced transcriptional upregulation. The 27nt RNA duplex matched to the partial sequence of the eNOS exon 24 was used as the RNA duplex control because no exonic sequence of the eNOS gene in these pGL3 reporter plasmids. As shown in Figure 2, addition of the 27nt RNA duplex significantly decreased the luciferase gene transcription efficiency in the plasmids containing 5 × 27nt repeats compared with the control (P < 0.01). However, this effect was not observed in the plasmid containing the SV40 enhancer, suggesting that the effect of the 27nt RNA duplex is specific for plasmids containing the 27nt repeats. Furthermore, β-actin (5 μg/mL) stimulated the luciferase gene transcription efficiency in both plasmids comparing to the controls treated by bovine serum albumin (Figure 2). When the 27nt duplex (50 nM) was added together with the β-actin, however, the stimulatory effects of the β-actin on the luciferase gene transcription efficiency in the plasmids containing the 5 × 27nt repeats was significantly suppressed (11.31 ± 1.08 × 10−6 versus 7.26 ± 0.90 × 10−6, P < 0.01) compared with the plasmids treated with β-actin alone. There was no such effect for the plasmids containing the SV40 enhancer. This indicates that the inhibitory effect of the 27nt RNA duplex on β-actin’s stimulation is specific to the plasmid containing the 5 × 27nt enhancer.
Figure 2.
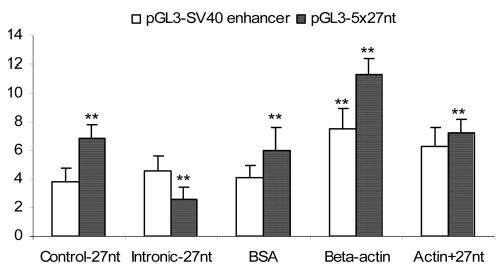
Interactive effect of exogenous 27nt RNA duplex and β-actin on the pGL3 transcription efficiency. Recombinant pGL3 plasmids containing either the SV40 enhancer or the 5 × 27nt enhancer at the 3′-end the luciferase coding sequence were subjected to the in vitro transcription reaction. When a control 27nt RNA duplex [a fraction of the eNOS exon 24 sequence (5′-GCGACGAGGTGCAGAACGCCCAGCAGC-3′), which has no complementary sequences in the recombinant pGL3 plasmids] was added to the in vitro transcription assay system, there was no effect on the luciferase mRNA levels. When the 27nt duplex (50 nM) complementary to the 27nt enhancer (5′-GAAGTCTAGACCTGCTGCAGGGGTGAG-3′) was added to the reaction, there was a significant reduction in the luciferase mRNA levels in the plasmid containing 5 × 27nt enhancer but not in the plasmids containing the SV40 enhancer. Addition of the β-actin (5.0 μg/mL) alone increased the transcription efficiency in both plasmids. When the β-actin was added together with the 27nt RNA duplex, transcription efficiency was significantly decreased for the plasmids containing a 5 × 27nt enhancer. It had no effect on the plasmids containing a SV40 enhancer. Experiments were repeated 3 times and results are presented as mean ± SEM. **P < 0.01 by the independent Student t test when compared with the control.
β-Actin Silencing Decreases eNOS Gene Expression in HAECs
To address our hypothesis that β-actin is essential in regulating the eNOS gene expression, we measured the eNOS gene expression at mRNA and protein levels in the cultured endothelial cells in which the β-actin was silenced by siRNA. The siRNA is homologous to the sequence of 974 to 992 of the β-actin mRNA (NM_001101, sense: 5′-GGCGGCACCACCATGTACC-3′, antisense: 5′-GGTACATGGTGGTGCCGCC-3′). A 19nt RNA duplex (sense 5′-GUCUGACAGUUACCAAUGC-3′, antisense 5′-GCAUUGGUAACUGUCAGAC-3′) from the mRNA sequence of the firefly luciferase gene (X65324) 153 to 171 was used as the control. As shown in Figure 3A, cells were transfected for 24 hours with the increasing concentrations of the β-actin specific siRNA. The eNOS mRNA levels were decreased by 50.4% when 50 nM of the β-actin siRNA was transfected (19.73 ± 1.30 versus 9.78 ± 1.05 × 10−3, P < 0.01). In the meantime, the β-actin mRNA was significantly decreased by 70.6% after the siRNA treatment (data not shown). In corresponding to the changes in the mRNA levels, Western blotting showed a dose-dependent decrease in eNOS protein levels (Figure 3B). In comparing with the controls, the eNOS enzyme activities were decreased by 43.1 ± 8.4% (P < 0.01) and 38.8 ± 7.8% (P < 0.01) for the cells treated by 50 nM and 100 nM β-actin siRNA, respectively. We further tested the time-dependent changes in the eNOS expression in HAECs treated with 50 nM of the β-actin specific siRNA. We found that the eNOS mRNA was decreased at as early as 18 hours after the β-actin siRNA transfection (Figure 3C). The decrease was continued to as low as 34% of the original values. The time-dependent changes in the eNOS mRNA levels were parallel by the eNOS protein changes (Figure 3D). Our data demonstrate that adequate supply of the β-actin may be essential for the eNOS expression in vivo.
Figure 3.
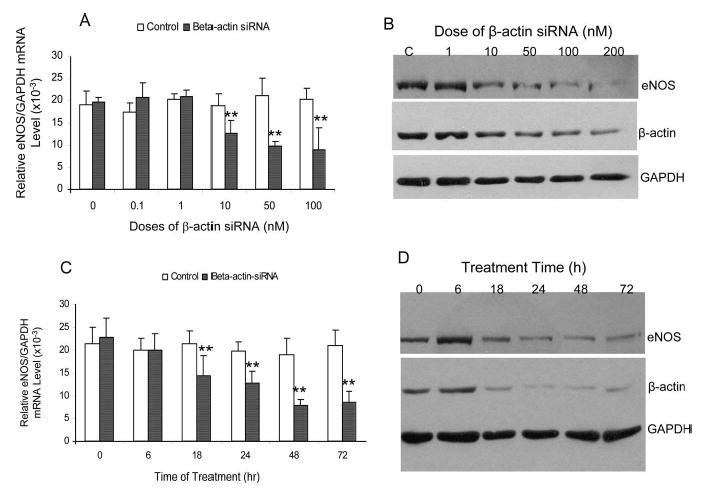
Effect of β-actin silencing on eNOS expression in cultured HAECs. HAECs were cultured up to 70% to 80% confluence. The β-actin specific siRNA was transfected to HAECs using Lipofectamine. For dose-dependent experiment, endothelial RNA and protein samples were collected after 24 or 48 hours of the siRNA treatment, respectively. We used a siRNA with the sequence homologous to a section of the luciferase coding region (sense 5′-GUCUGACAGUUACCAAUGC-3′, antisense 5′-GCAUUGGUAACUGUCAGAC-3′) as the nonspecific control because no luciferase was expressed in endothelial cells. A, Dose-dependent effect of β-actin siRNA on eNOS mRNA expression by the quantitative real-time reverse-transcription polymerase chain reaction. B, Dose-dependent effect of β-actin siRNA on eNOS protein levels by Western blotting. C, Time-dependent changes in eNOS mRNA levels when endothelial cells were treated with 50 nM β-actin siRNA. D, Time-dependent changes in eNOS protein levels when endothelial cells were treated with 50 nM β-actin siRNA. The levels of GAPDH mRNA or protein were used as the endogenous control for the adjustment of the eNOS mRNA and protein levels in cells treated with β-actin siRNA. **P < 0.01 by independent Student t test when compared with the endothelial cells treated with the luciferase specific siRNA as a negative control.
β-Actin Overexpression Increases eNOS Expression in HAECs
As presented, β-actin silencing by the specific siRNA resulted in a significant reduction in the eNOS gene expression. We then examined whether overexpressing β-actin would lead to an increased eNOS expression. We transfected the HAECs with the pAcGFP-Actin plasmids containing the human β-actin complete coding sequence. We used the pAcGFP-C1 containing no actin as the plasmid control. Figure 4A shows 2 representative images of the GFP-Actin fusion protein (green fluorescence light) expressed in HAECs 48 hours after the pAcGFP-Actin plasmid transfection. The nuclei were stained in red by the Propidium Iodide dye. In each image, there are 2 cells: one represents effective transfection of the β-actin-GFP plasmids (in green); another represents untransfected cell showing only the nucleus stained in red by the Propidium Iodide. In the upper image, most of the fusion proteins were located in the cytoplasm, whereas the bottom image indicated that certain fusion proteins entered into the nucleus.
Figure 4.
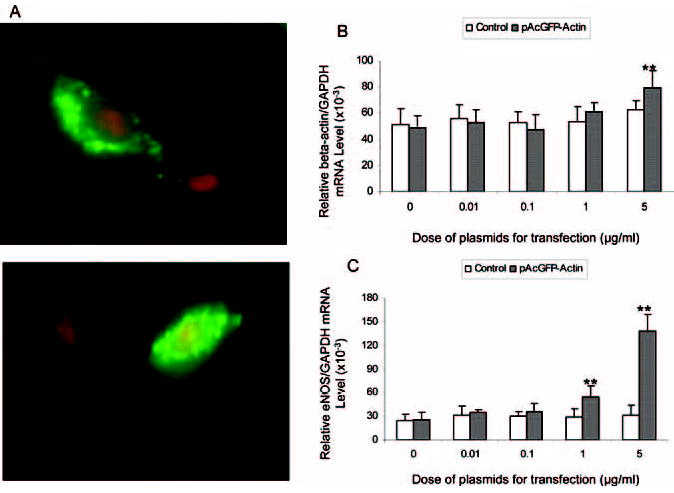
Effect of β-actin overexpression on eNOS mRNA and protein levels in cultured HAECs. A, HAECs were transfected with pAcGFP-Actin (green fluorescence) (5.0 μg/mL) for 48 hours before counterstained with propidium iodide (red fluorescence). The upper image shows that the β-actin (green) is located mainly in the cytoplasm, whereas the lower image shows the nuclear presence of the transfected exogenous β-actin. There was an untransfected cell as the control in both images. B and C, HAECs were transfected with denoted concentrations of the pAcGFP-Actin or pAcGFP-C1 (control) plasmids. After 24 hours of transfection, the total RNA was collected for the mRNA measurements by the quantitative real-time reverse-transcription polymerase chain reaction. GAPHD was used as the endogenous standard for the relative eNOS or β-actin mRNA levels. β-actin mRNA was elevated only at the highest dose (5.0 μg/mL) of the pAcGFP-Actin plasmids (B). The eNOS mRNA was upregulated by 1.0 μg/mL and 5.0 μg/mL pAcGFP-Actin plasmids used in the transfection (C). No changes in the eNOS or β-actin mRNA levels were observed in cells transfected with the control plasmid (B and C).
We further measured the mRNA levels of β-actin and eNOS using GAPDH as the internal control. As shown in Figure 4B, β-actin mRNA levels increased in the cells transfected with the highest dose of pAcGFP-Actin (5.0 μg/mL) (79.66 ± 12.79 versus 49.04 ± 8.78 × 10−3, P < 0.01). At the lower dose, however, changes in β-actin mRNA was not obvious (Figure 4B). This was probably because of a relatively small contribution of the transfected exogenous β-actin to the large pool of the already abundantly expressed endogenous β-actin mRNA. As shown in Figure 4C, pAcGFP-Actin transfection at the dose of 5.0 μg/mL also resulted in a 4.5-fold increase in the eNOS mRNA levels compared with the control (transfection with the pAcGFP-C1) (138.70 ± 20.78 versus 25.38 ± 9.45 × 10−3, P < 0.01). The increase in eNOS mRNA corresponded with the increase in the eNOS protein levels (data not shown) and the eNOS enzyme activities (232.5 ± 31.2% versus 100 ± 13.4%, P < 0.01). At a lower dose of the pAcGFP-Actin (1.0 μg/mL), the eNOS mRNA level was also significantly elevated compared with the cells transfected with control vector (54.41 ± 13.97 versus 25.38 ± 9.45 × 10−3, P < 0.01. Figure 4C). At this low dose, however, the β-actin mRNA was only slightly increased (61.10 ± 6.80 versus 49.04 ± 8.78 × 10−3, P > 0.05; Figure 4B).
Discussion
In the present study, we have reported an upregulatory effect of the nuclear actin on the eNOS expression; this upregulatory effect is dependent on the presence of the 27nt repeat element in intron 4. Our experiments have further shown the presence of the monomeric actin within endothelial nucleus. When the actin expression was inhibited by the actin specific siRNA, the eNOS expression was reduced. When the actin expression was enhanced by the overexpressing vector, the eNOS expression was increased. As far as we are aware, this is the first report on the actin-based novel eNOS regulatory mechanism.
Actin is generally regarded as a structural protein until its nuclear presence was defined recently by several studies.8–11 Whereas actin has no nuclear localization signal for direct nuclear import, cofilin (containing nuclear localization signal) appears to act as an active carrier for actin’s nuclear translocation. Once inside the nucleus, actin can be exported via 2 highly conserved and functional nuclear export signals, which are found in all actins.12 It has been suggested that nuclear actin is in a soluble monomeric form rather than a filament polymeric form as that in the cytoskeleton.9,11 Under stress, actin may translocate to nuclei to function as a stress sensor.9,13,14 Because endothelial NO is the key molecule for endothelial functional integrity, eNOS becomes a logical target for the response to stress. However, the transcriptional activity of the nuclear actin is unlikely to be confined to the eNOS only. Our experiments have also shown the upregulatory effects on the SV40 enhancer-driven expression. Nuclear actin may upregulate a group of genes in response to the “stress.” The stress and response genes may be cell specific, and are influenced by the metabolic status of the cells at the time of stress.
Although it is far from clear how a cytoplasmic structural protein could turn into a nuclear transcriptional factor, several possible mechanisms could be responsible for the regulatory effect of the nuclear actin on the eNOS expression. Nuclear actin could: (1) play a direct role in RNA transcription as a part of pre-initiation complex with the RNA polymerase II; (2) be involved in the RNA processing and export via its interaction with eukaryotic initiation factor-5A and small nuclear ribonucleoprotein; (3) participate in chromatin-remodeling, eg, association with BRG-associated factor complex, which is a mammalian chromatin-remodeling complex; and (4) be part of the nucleoskeleton9,10,15–17 and facilitate the eNOS mRNA shuttling. Because the actin’s effect is 27nt-dependent and the 27nt repeat element in the eNOS intron 4 functions as an enhancer,7 actin is more likely to be directly involved in the RNA transcriptional complex for its transcriptional effect. However, the fact that the 27nt RNA duplex can interfere with the effect of actin also suggests a possibility of functional interaction in pre-mRNA processing. More detailed experiments are needed to confirm this hypothesis and to explore the mechanisms. In addition, identification of the amino acid sequence in nuclear actin responsible for the effect will be of great value to the development of pharmaceutical agents regulating eNOS expression.
In summary, our study, for the first time to our knowledge, shows a regulatory effect of the nuclear actin on the eNOS expression; this regulatory effect depends on the presence of the 27nt element as an enhancer. Apart from statin, no pharmaceutical agents at this stage can directly upregulate eNOS expression.18–21 Our study reveals a novel pathway that could facilitate discoveries of agents, eg, actin-derived short active peptides, to specifically regulate eNOS expression, hence bioavailability of vascular NO.
Supplementary Material
Acknowledgments
The work is supported by a grant from National Institutes of Health (R01-HL066053). Dr X.L. Wang is an American Heart Association Established Investigator.
References
- 1.Moncada S, Palmer RM, Higgs EA. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991;43:109–142. [PubMed] [Google Scholar]
- 2.Sessa WC. eNOS at a glance. J Cell Sci. 2004;117:2427–2429. doi: 10.1242/jcs.01165. [DOI] [PubMed] [Google Scholar]
- 3.Tai SC, Robb GB, Marsden PA. Endothelial nitric oxide synthase: a new paradigm for gene regulation in the injured blood vessel. Arterioscler Thromb Vasc Biol. 2004;24:405–412. doi: 10.1161/01.ATV.0000109171.50229.33. Epub 2003 Dec 1. [DOI] [PubMed] [Google Scholar]
- 4.Wang XL, Sim AS, Badenhop RF, McCredie RM, Wilcken DE. A smoking-dependent risk of coronary artery disease associated with a polymorphism of the endothelial nitric oxide synthase gene. Nat Med. 1996;2:41–45. doi: 10.1038/nm0196-41. [DOI] [PubMed] [Google Scholar]
- 5.Wang XL, Mahaney MC, Sim AS, Wang J, Blangero J, Almasy L, Badenhop RB, Wilcken DE. Genetic contribution of the endothelial constitutive nitric oxide synthase gene to plasma nitric oxide levels. Arterioscler Thromb Vasc Biol. 1997;17:3147–3153. doi: 10.1161/01.atv.17.11.3147. [DOI] [PubMed] [Google Scholar]
- 6.Wang XL, Sim AS, Wang MX, Murrell GA, Trudinger B, Wang J. Genotype dependent and cigarette specific effects on endothelial nitric oxide synthase gene expression and enzyme activity. FEBS Lett. 2000;471:45–50. doi: 10.1016/s0014-5793(00)01356-9. [DOI] [PubMed] [Google Scholar]
- 7.Wang J, Dudley D, Wang XL. Haplotype-specific effects on endothelial NO synthase promoter efficiency: modifiable by cigarette smoking. Arterioscler Thromb Vasc Biol. 2002;22:e1–4. doi: 10.1161/01.ATV.0000016248.51577.1F. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Franke WW. Actin’s many actions start at the genes. Nat Cell Biol. 2004;6:1013–1014. doi: 10.1038/ncb1104-1013. [DOI] [PubMed] [Google Scholar]
- 9.Bettinger BT, Gilbert DM, Amberg DC. Actin up in the nucleus. Nat Rev Mol Cell Biol. 2004;5:410–415. doi: 10.1038/nrm1370. [DOI] [PubMed] [Google Scholar]
- 10.Kiseleva E, Drummond SP, Goldberg MW, Rutherford SA, Allen TD, Wilson KL. Actin- and protein-4.1-containing filaments link nuclear pore complexes to subnuclear organelles in Xenopus oocyte nuclei. J Cell Sci. 2004;117:2481–2490. doi: 10.1242/jcs.01098. Epub 2004 May 05. [DOI] [PubMed] [Google Scholar]
- 11.Gonsior SM, Platz S, Buchmeier S, Scheer U, Jockusch BM, Hinssen H. Conformational difference between nuclear and cytoplasmic actin as detected by a monoclonal antibody. J Cell Sci. 1999;112:797–809. doi: 10.1242/jcs.112.6.797. [DOI] [PubMed] [Google Scholar]
- 12.Wada A, Fukuda M, Mishima M, Nishida E. Nuclear export of actin: a novel mechanism regulating the subcellular localization of a major cytoskeletal protein. EMBO J. 1998;17:1635–1641. doi: 10.1093/emboj/17.6.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fukui Y, Katsumaru H. Nuclear actin bundles in Amoeba, Dictyostelium and human HeLa cells induced by dimethyl sulfoxide. Exp Cell Res. 1979;120:451–455. doi: 10.1016/0014-4827(79)90412-9. [DOI] [PubMed] [Google Scholar]
- 14.Fukui Y, Katsumaru H. Dynamics of nuclear actin bundle induction by dimethyl sulfoxide and factors affecting its development. J Cell Biol. 1980;84:131–140. doi: 10.1083/jcb.84.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hofmann WA, Stojiljkovic L, Fuchsova B, Vargas GM, Mavrommatis E, Philimonenko V, Kysela K, Goodrich JA, Lessard JL, Hope TJ, Hozak P, de Lanerolle P. Actin is part of pre-initiation complexes and is necessary for transcription by RNA polymerase II. . Nat Cell Biol. 2004;6:1094–1101. doi: 10.1038/ncb1182. Epub 2004 Oct 24. [DOI] [PubMed] [Google Scholar]
- 16.Fomproix N, Percipalle P. An actin-myosin complex on actively transcribing genes. Exp Cell Res. 2004;294:140–148. doi: 10.1016/j.yexcr.2003.10.028. [DOI] [PubMed] [Google Scholar]
- 17.Percipalle P, Fomproix N, Kylberg K, Miralles F, Bjorkroth B, Daneholt B, Visa N. An actin-ribonucleoprotein interaction is involved in transcription by RNA polymerase II. Proc Natl Acad Sci U S A. 2003;100:6475–6480. doi: 10.1073/pnas.1131933100. Epub 2003 May 12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Landmesser U, Engberding N, Bahlmann FH, Schaefer A, Wiencke A, Heineke A, Spiekermann S, Hilfiker-Kleiner D, Templin C, Kotlarz D, Mueller M, Fuchs M, Hornig B, Haller H, Drexler H. Statin-induced improvement of endothelial progenitor cell mobilization, myocardial neovascularization, left ventricular function, and survival after experimental myocardial infarction requires endothelial nitric oxide synthase. Circulation. 2004;110:1933–1939. doi: 10.1161/01.CIR.0000143232.67642.7A. [DOI] [PubMed] [Google Scholar]
- 19.Hernandez-Perera O, Perez-Sala D, Navarro-Antolin J, Sanchez-Pascuala R, Hernandez G, Diaz C, Lamas S. Effects of the 3-hydroxy-3-methylglutaryl-CoA reductase inhibitors, atorvastatin and simvastatin, on the expression of endothelin-1 and endothelial nitric oxide synthase in vascular endothelial cells. J Clin Invest. 1998;101:2711–2719. doi: 10.1172/JCI1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Beckman JA, Liao JK, Hurley S, Garrett LA, Chui D, Mitra D, Creager MA. Atorvastatin restores endothelial function in normocholesterolemic smokers independent of changes in low-density lipoprotein. Circ Res. 2004;95:217–223. doi: 10.1161/01.RES.0000134628.96682.9b. Epub 2004 Jun 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brouet A, Sonveaux P, Dessy C, Moniotte S, Balligand JL, Feron O. Hsp90 and caveolin are key targets for the proangiogenic nitric oxide-mediated effects of statins. Circ Res. 2001;89:866–873. doi: 10.1161/hh2201.100319. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


