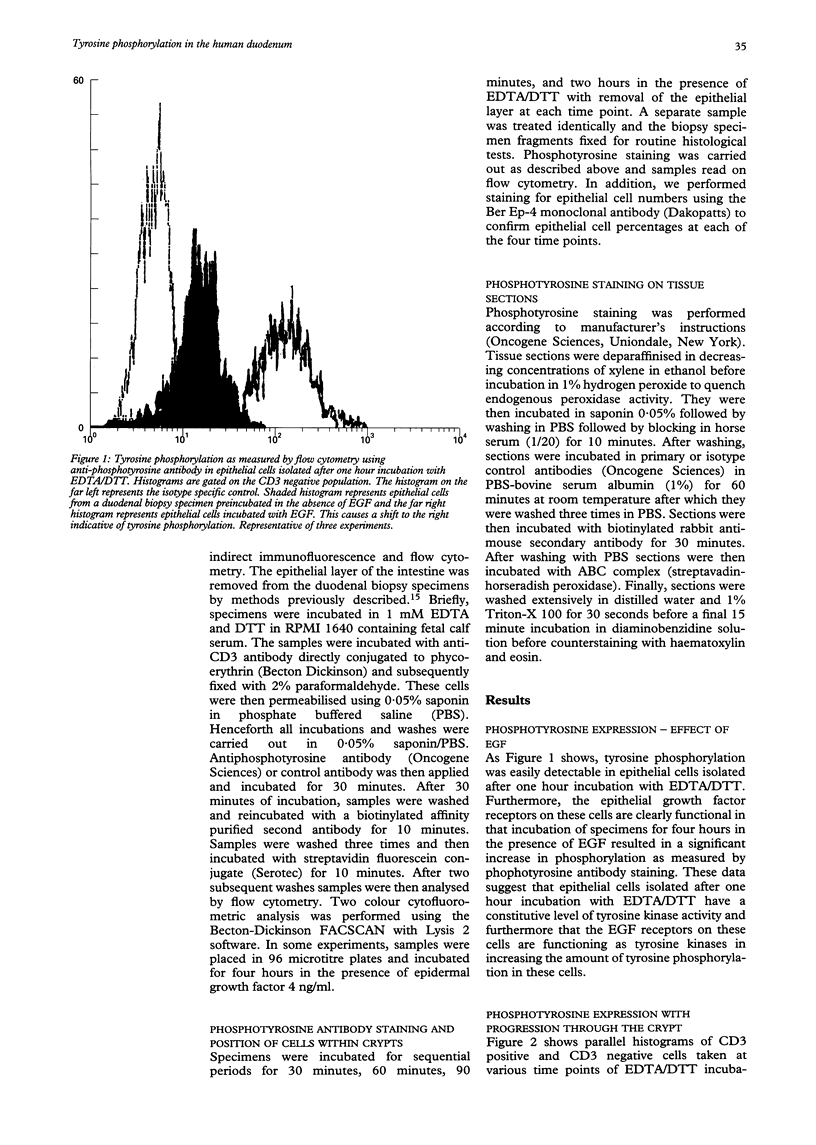
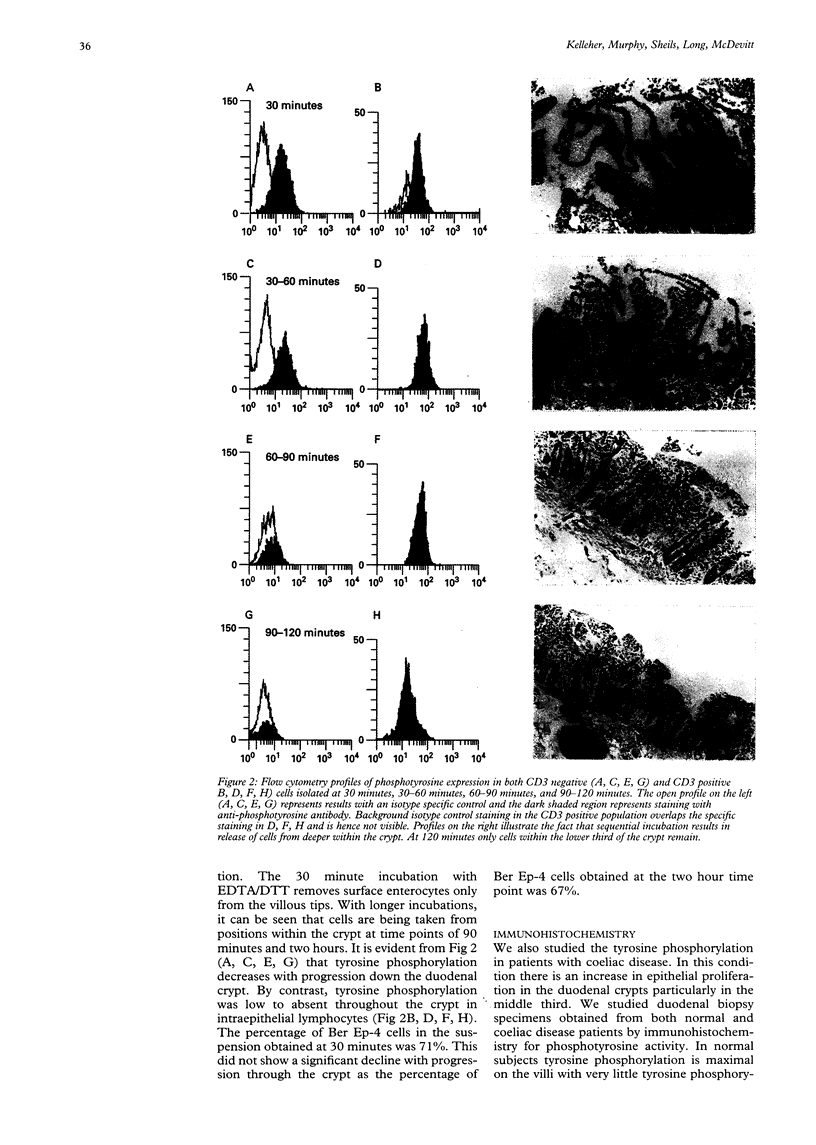
Abstract
Many growth factor receptors including the epidermal growth factor receptor function through tyrosine kinase activity. The aim of this study was to examine the constitutive level of tyrosine phosphorylation in the normal duodenum and in the hyperproliferative coeliac duodenum. A flow cytometric assay was devised using monoclonal antibody to phosphorylated (but not native) tyrosine residues to determine the levels of tyrosine phosphorylation in both CD3 positive intraepithelial lymphocytes and CD3 negative epithelial cells obtained by EDTA treatment of endoscopically obtained duodenal biopsy specimens. In addition, immunohistochemistry was performed on 18 formalin fixed coeliac duodenal biopsy specimens and eight control specimens. Tyrosine phosphorylation could be detected by flow cytometry on duodenal enterocytes and this expression was up regulated by pretreatment with epidermal growth factor. Tyrosine phosphorylation decreased with progression from the villus to the crypt, however, and was virtually undetectable on crypt enterocytes. Immunohistochemistry of the coeliac duodenum showed virtually absent tyrosine phosphorylation in the crypt. Increased tyrosine phosphorylation was detected in the infiltrating T cells. In conclusion, tyrosine phosphorylation in the duodenum is confined to the non-proliferative villous epithelium and is virtually undetectable in the proliferative crypt compartment. These findings suggest that tyrosine kinase activity is not a significant factor in the regulation of crypt cell proliferation in the human duodenum either in normal subjects or in coeliac disease patients.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Chen W. S., Lazar C. S., Poenie M., Tsien R. Y., Gill G. N., Rosenfeld M. G. Requirement for intrinsic protein tyrosine kinase in the immediate and late actions of the EGF receptor. 1987 Aug 27-Sep 2Nature. 328(6133):820–823. doi: 10.1038/328820a0. [DOI] [PubMed] [Google Scholar]
- Ferris D. K., Willette-Brown J., Ortaldo J. R., Farrar W. L. IL-2 regulation of tyrosine kinase activity is mediated through the p70-75 beta-subunit of the IL-2 receptor. J Immunol. 1989 Aug 1;143(3):870–876. [PubMed] [Google Scholar]
- Gallagher R. B., Kelly C. P., Neville S., Sheils O., Weir D. G., Feighery C. F. Complement activation within the coeliac small intestine is localised to Brunner's glands. Gut. 1989 Nov;30(11):1568–1573. doi: 10.1136/gut.30.11.1568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad R. A., Wilson T. J., Lenton W., Gregory H., McCullagh K. G., Wright N. A. Intravenous but not intragastric urogastrone-EGF is trophic to the intestine of parenterally fed rats. Gut. 1987 May;28(5):573–582. doi: 10.1136/gut.28.5.573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodlad R. A., Wilson T. J., Lenton W., Gregory H., McCullough K. G., Wright N. A. Urogastrone-epidermal growth factor is trophic to the intestinal epithelium of parenterally fed rats. Experientia. 1985 Sep 15;41(9):1161–1163. doi: 10.1007/BF01951708. [DOI] [PubMed] [Google Scholar]
- Goodlad R. A., Wright N. A. Growth control factors in the gastrointestinal tract. Baillieres Clin Gastroenterol. 1990 Mar;4(1):97–118. doi: 10.1016/0950-3528(90)90041-e. [DOI] [PubMed] [Google Scholar]
- Huhn R. D., Posner M. R., Rayter S. I., Foulkes J. G., Frackelton A. R., Jr Cell lines and peripheral blood leukocytes derived from individuals with chronic myelogenous leukemia display virtually identical proteins phosphorylated on tyrosine residues. Proc Natl Acad Sci U S A. 1987 Jul;84(13):4408–4412. doi: 10.1073/pnas.84.13.4408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunter T. Protein-tyrosine phosphatases: the other side of the coin. Cell. 1989 Sep 22;58(6):1013–1016. doi: 10.1016/0092-8674(89)90496-0. [DOI] [PubMed] [Google Scholar]
- Koretzky G. A., Kohmetscher M. A., Kadleck T., Weiss A. Restoration of T cell receptor-mediated signal transduction by transfection of CD45 cDNA into a CD45-deficient variant of the Jurkat T cell line. J Immunol. 1992 Aug 15;149(4):1138–1142. [PubMed] [Google Scholar]
- Lowes J. R., Priddle J. D., Jewell D. P. Production of epithelial cell growth factors by lamina propria mononuclear cells. Gut. 1992 Jan;33(1):39–43. doi: 10.1136/gut.33.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald T. T. The role of activated T lymphocytes in gastrointestinal disease. Clin Exp Allergy. 1990 May;20(3):247–252. doi: 10.1111/j.1365-2222.1990.tb02679.x. [DOI] [PubMed] [Google Scholar]
- Madrigal L., Lynch S., Feighery C., Weir D., Kelleher D., O'Farrelly C. Flow cytometric analysis of surface major histocompatibility complex class II expression on human epithelial cells prepared from small intestinal biopsies. J Immunol Methods. 1993 Feb 3;158(2):207–214. doi: 10.1016/0022-1759(93)90216-t. [DOI] [PubMed] [Google Scholar]
- Peraldi P., Hauguel-de Mouzon S., Alengrin F., Van Obberghen E. Dephosphorylation of human insulin-like growth factor I (IGF-I) receptors by membrane-associated tyrosine phosphatases. Biochem J. 1992 Jul 1;285(Pt 1):71–78. doi: 10.1042/bj2850071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peyron J. F., Verma S., de Waal Malefyt R., Sancho J., Terhorst C., Spits H. The CD45 protein tyrosine phosphatase is required for the completion of the activation program leading to lymphokine production in the Jurkat human T cell line. Int Immunol. 1991 Dec;3(12):1357–1366. doi: 10.1093/intimm/3.12.1357. [DOI] [PubMed] [Google Scholar]
- Pollack P. F., Goda T., Colony P. C., Edmond J., Thornburg W., Korc M., Koldovský O. Effects of enterally fed epidermal growth factor on the small and large intestine of the suckling rat. Regul Pept. 1987 Mar;17(3):121–132. doi: 10.1016/0167-0115(87)90021-8. [DOI] [PubMed] [Google Scholar]
- Preis P. N., Waldman F. M., Frackelton A. R., Jr, Saya H., Levin V. A. Protein-tyrosine kinase activity and pp60v-src expression in whole cells measured by flow cytometry. Cancer Res. 1988 Aug 15;48(16):4633–4638. [PubMed] [Google Scholar]
- Sengupta A., Liu W. K., Yeung Y. G., Yeung D. C., Frackelton A. R., Jr, Stanley E. R. Identification and subcellular localization of proteins that are rapidly phosphorylated in tyrosine in response to colony-stimulating factor 1. Proc Natl Acad Sci U S A. 1988 Nov;85(21):8062–8066. doi: 10.1073/pnas.85.21.8062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Streuli M., Krueger N. X., Ariniello P. D., Tang M., Munro J. M., Blattler W. A., Adler D. A., Disteche C. M., Saito H. Expression of the receptor-linked protein tyrosine phosphatase LAR: proteolytic cleavage and shedding of the CAM-like extracellular region. EMBO J. 1992 Mar;11(3):897–907. doi: 10.1002/j.1460-2075.1992.tb05128.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas D. M., Nasim M. M., Gullick W. J., Alison M. R. Immunoreactivity of transforming growth factor alpha in the normal adult gastrointestinal tract. Gut. 1992 May;33(5):628–631. doi: 10.1136/gut.33.5.628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trowbridge I. S. CD45. A prototype for transmembrane protein tyrosine phosphatases. J Biol Chem. 1991 Dec 15;266(35):23517–23520. [PubMed] [Google Scholar]
- Ullrich A., Schlessinger J. Signal transduction by receptors with tyrosine kinase activity. Cell. 1990 Apr 20;61(2):203–212. doi: 10.1016/0092-8674(90)90801-k. [DOI] [PubMed] [Google Scholar]
- Walker-Smith J. A., Phillips A. D., Walford N., Gregory H., Fitzgerald J. D., MacCullagh K., Wright N. A. Intravenous epidermal growth factor/urogastrone increases small-intestinal cell proliferation in congenital microvillous atrophy. Lancet. 1985 Nov 30;2(8466):1239–1240. doi: 10.1016/s0140-6736(85)90762-7. [DOI] [PubMed] [Google Scholar]
- Weaver L. T., Walker W. A. Epidermal growth factor and the developing human gut. Gastroenterology. 1988 Mar;94(3):845–847. doi: 10.1016/0016-5085(88)90263-6. [DOI] [PubMed] [Google Scholar]
- Williams L. T. Signal transduction by the platelet-derived growth factor receptor. Science. 1989 Mar 24;243(4898):1564–1570. doi: 10.1126/science.2538922. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]
- Wright N., Watson A., Morley A., Appleton D., Marks J. Cell kinetics in flat (avillous) mucosa of the human small intestine. Gut. 1973 Sep;14(9):701–710. doi: 10.1136/gut.14.9.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi T., Cleveland J. L., Ihle J. N. Identification of novel protein tyrosine phosphatases of hematopoietic cells by polymerase chain reaction amplification. Blood. 1991 Nov 1;78(9):2222–2228. [PubMed] [Google Scholar]