Abstract
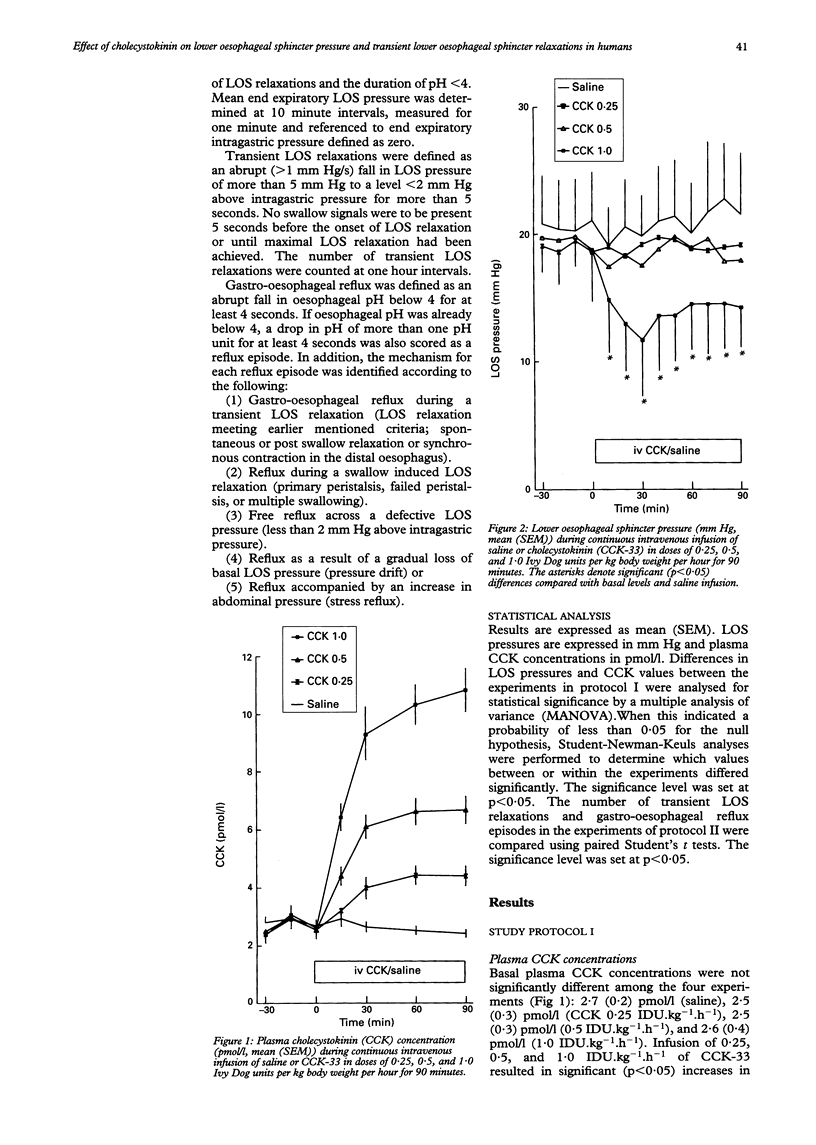
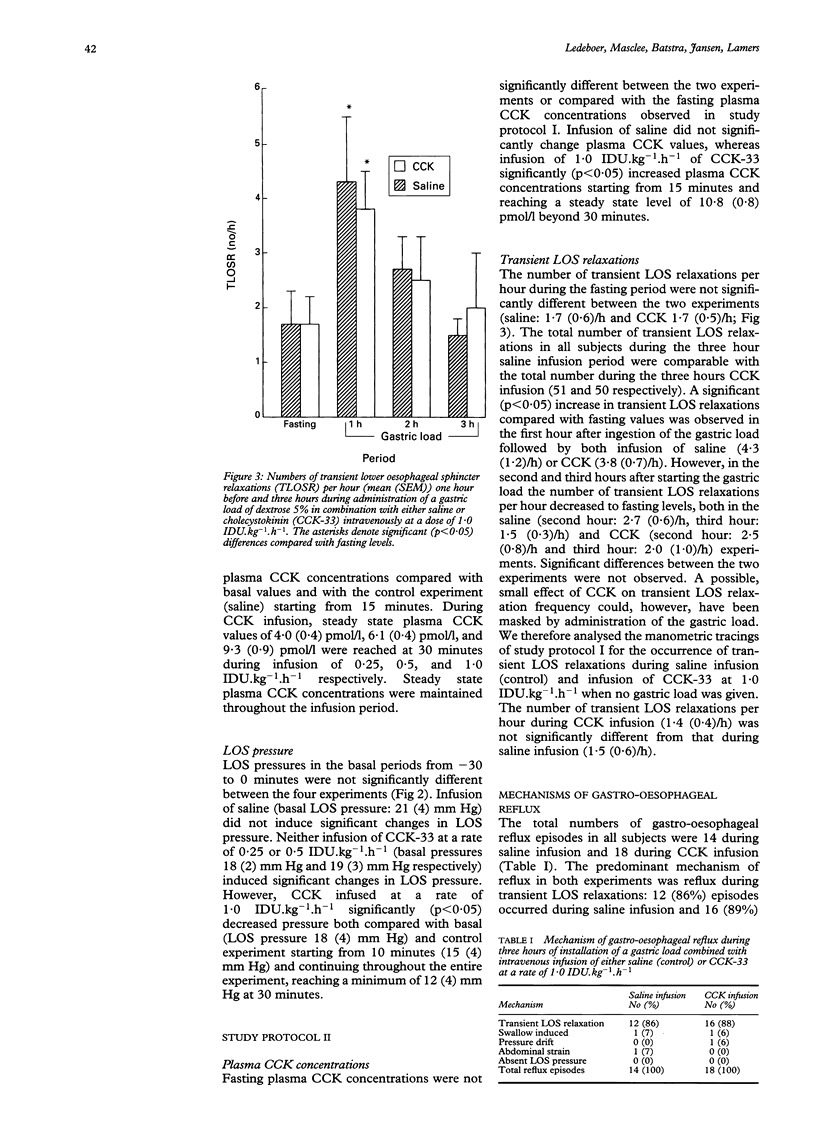
The effect of cholecystokinin (CCK) on the lower oesophageal sphincter (LOS) pressure, frequency of transient LOS relaxations, and the number of reflux episodes was investigated in six healthy subjects. LOS pressure was recorded on four separate occasions during continuous intravenous infusion of either saline or CCK-33 in doses of 0.25, 0.5, or 1.0 Ivy Dog units per kg body weight per hour (IDU.kg-1.h-1) for 90 minutes. Plasma CCK concentrations did not change during saline infusion, but increased significantly from 2.5 (0.3) pmol/l to steady state levels of 4.0 (0.4) pmol/l, 6.1 (0.4) pmol/l, and 9.3 (0.9) pmol/l respectively starting from 30 minutes. LOS pressure did not change significantly during infusion of saline or of CCK-33 at doses of 0.25 or 0.5 IDU.kg-1.h-1. However, a significant (p < 0.05) reduction in LOS pressure to a minimum level of 12 (4) mm Hg at 30 minutes compared with basal level (18 (4) mm Hg) and compared with saline was observed during infusion of CCK-33 at a dose of 1.0 IDU.kg-1.h-1. In addition, oesophageal motility and pH were recorded simultaneously in these six subjects on two separate occasions one hour before (fasting) and three hours during administration of a gastric load (dextrose 5%, pH 3) combined with continuous intravenous infusion of saline or CCK-33 at a dose of 1.0 IDU,kg-1.h-1. Plasma CCK concentrations did not change during the gastric load combined with saline, but increased significantly to a steady state level of 10.8 (0.8) pmol/l during intravenous infusion of CCK. The number of transient LOS relaxations increased significantly in the first hour during administration of the gastric load compared with fasting levels, both during saline infusion (fasting: 1.7 (0.6)/h, 1st hour: 4.3 (1.2)/h) and during CCK infusion (fasting: 1.7 (0.5)/h, 1st hour: 3.8 (0.7)/h). In the second and third hours the number of transient LOS relaxations fell to fasting levels in both experiments. No significant differences were observed in the number and type of transient LOS relaxations, mechanism of gastro-oesophageal reflux, or duration of acid exposure between the two experiments. It is concluded that in healthy subjects infusion of CCK-33 in a dose of 1.0 IDU.kg-1.h-1 significantly reduces LOS pressure but does not affect the frequency of transient LOS relaxations or acid exposure time during a continuous liquid gastric load.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babka J. C., Castell D. O. On the genesis of heartburn. The effects of specific foods on the lower esophageal sphincter. Am J Dig Dis. 1973 May;18(5):391–397. doi: 10.1007/BF01071988. [DOI] [PubMed] [Google Scholar]
- Becker D. J., Sinclair J., Castell D. O., Wu W. C. A comparison of high and low fat meals on postprandial esophageal acid exposure. Am J Gastroenterol. 1989 Jul;84(7):782–786. [PubMed] [Google Scholar]
- Brazer S. R., Borislow D. S., Liddle R. A. Cholecystokinin is not a major hormonal regulator of lower esophageal sphincter pressure. Gastroenterology. 1990 Sep;99(3):641–645. doi: 10.1016/0016-5085(90)90949-2. [DOI] [PubMed] [Google Scholar]
- Dent J. A new technique for continuous sphincter pressure measurement. Gastroenterology. 1976 Aug;71(2):263–267. [PubMed] [Google Scholar]
- Dent J., Dodds W. J., Friedman R. H., Sekiguchi T., Hogan W. J., Arndorfer R. C., Petrie D. J. Mechanism of gastroesophageal reflux in recumbent asymptomatic human subjects. J Clin Invest. 1980 Feb;65(2):256–267. doi: 10.1172/JCI109667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dent J., Holloway R. H., Toouli J., Dodds W. J. Mechanisms of lower oesophageal sphincter incompetence in patients with symptomatic gastrooesophageal reflux. Gut. 1988 Aug;29(8):1020–1028. doi: 10.1136/gut.29.8.1020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dodds W. J., Dent J., Hogan W. J., Helm J. F., Hauser R., Patel G. K., Egide M. S. Mechanisms of gastroesophageal reflux in patients with reflux esophagitis. N Engl J Med. 1982 Dec 16;307(25):1547–1552. doi: 10.1056/NEJM198212163072503. [DOI] [PubMed] [Google Scholar]
- Fisher R. S., DiMarino A. J., Cohen S. Mechanism of cholecystokinin inhibition of lower esophageal sphincter pressure. Am J Physiol. 1975 May;228(5):1469–1473. doi: 10.1152/ajplegacy.1975.228.5.1469. [DOI] [PubMed] [Google Scholar]
- Fried M., Mayer E. A., Jansen J. B., Lamers C. B., Taylor I. L., Bloom S. R., Meyer J. H. Temporal relationships of cholecystokinin release, pancreatobiliary secretion, and gastric emptying of a mixed meal. Gastroenterology. 1988 Nov;95(5):1344–1350. doi: 10.1016/0016-5085(88)90371-x. [DOI] [PubMed] [Google Scholar]
- Holloway R. H., Hongo M., Berger K., McCallum R. W. Gastric distention: a mechanism for postprandial gastroesophageal reflux. Gastroenterology. 1985 Oct;89(4):779–784. doi: 10.1016/0016-5085(85)90572-4. [DOI] [PubMed] [Google Scholar]
- Hopman W. P., Jansen J. B., Lamers C. B. Comparative study of the effects of equal amounts of fat, protein, and starch on plasma cholecystokinin in man. Scand J Gastroenterol. 1985 Sep;20(7):843–847. doi: 10.3109/00365528509088832. [DOI] [PubMed] [Google Scholar]
- Jansen J. B., Lamers C. B. Molecular forms of cholecystokinin in plasma from normal and gastrectomized human subjects following a fat meal. Peptides. 1987 Sep-Oct;8(5):801–805. doi: 10.1016/0196-9781(87)90062-3. [DOI] [PubMed] [Google Scholar]
- Jansen J. B., Lamers C. B. Radioimmunoassay of cholecystokinin in human tissue and plasma. Clin Chim Acta. 1983 Jul 15;131(3):305–316. doi: 10.1016/0009-8981(83)90100-6. [DOI] [PubMed] [Google Scholar]
- Liddle R. A., Goldfine I. D., Rosen M. S., Taplitz R. A., Williams J. A. Cholecystokinin bioactivity in human plasma. Molecular forms, responses to feeding, and relationship to gallbladder contraction. J Clin Invest. 1985 Apr;75(4):1144–1152. doi: 10.1172/JCI111809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mittal R. K., McCallum R. W. Characteristics and frequency of transient relaxations of the lower esophageal sphincter in patients with reflux esophagitis. Gastroenterology. 1988 Sep;95(3):593–599. doi: 10.1016/s0016-5085(88)80003-9. [DOI] [PubMed] [Google Scholar]
- Mittal R. K., McCallum R. W. Characteristics of transient lower esophageal sphincter relaxation in humans. Am J Physiol. 1987 May;252(5 Pt 1):G636–G641. doi: 10.1152/ajpgi.1987.252.5.G636. [DOI] [PubMed] [Google Scholar]
- Nebel O. T., Castell D. O. Lower esophageal sphincter pressure changes after food ingestion. Gastroenterology. 1972 Nov;63(5):778–783. [PubMed] [Google Scholar]
- Resin H., Stern D. H., Sturdevant R. A., Isenberg J. I. Effect of the C-terminal octapeptide of cholecystokinin on lower esophageal sphincter pressure in man. Gastroenterology. 1973 May;64(5):946–949. [PubMed] [Google Scholar]
- Sturdevant R. A., Kun T. Interaction of pentagastrin and the octapeptide of cholecystokinin on the human lower oesophageal sphincter. Gut. 1974 Sep;15(9):700–702. doi: 10.1136/gut.15.9.700. [DOI] [PMC free article] [PubMed] [Google Scholar]


