Abstract
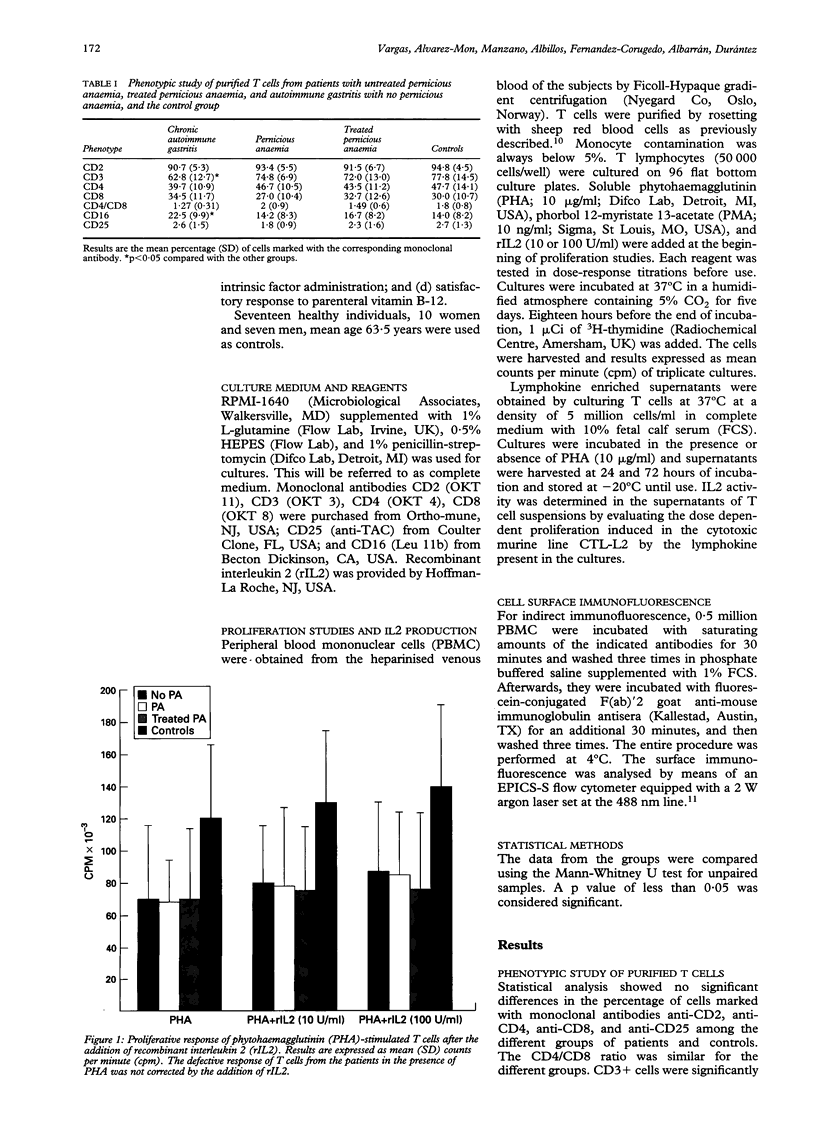
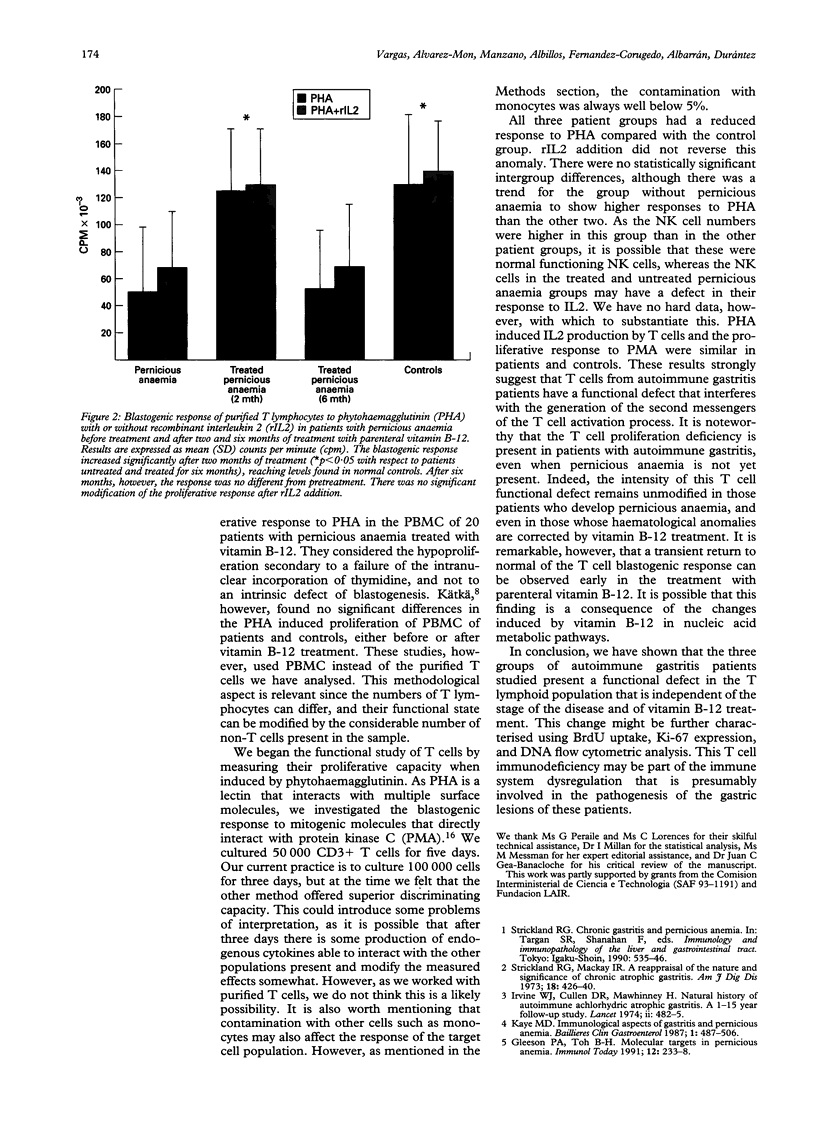
The functional response and phenotypic characterisation of peripheral blood T cells were studied in 41 patients with autoimmune gastritis--nine patients with autoimmune gastritis alone, 11 with untreated pernicious anaemia, and 21 with resolved pernicious anaemia who were taking vitamin B-12. Phenotypic analysis showed no changes in the CD4/CD8 ratio in any group of patients. CD3+ cells were significantly decreased and CD16+ cells were significantly increased in patients with autoimmune gastritis alone. Phytohaemagglutinin induced T cell proliferation, with or without interleukin 2, was reduced in the three groups. T cell proliferation induced by phorbol myristate acetate was normal. Interleukin 2 production of phytohaemagglutinin-stimulated lymphocytes was normal in the three groups. Five patients with pernicious anaemia treated with vitamin B-12 were followed and persistent hypoproliferation of T cells in response to phytohaemagglutinin was observed. The follow up study of the phenotype of these patients showed a significant increase of the CD2+ CD3- lymphocyte population after six months' treatment. In conclusion, the three groups of autoimmune gastritis patients studied have a functional defect in T cells that is independent of B-12 treatment and of the presence of pernicious anaemia.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alvarez de Mon M., Casas J., Laguna R., Toribio M. L., de Landázuri M. O., Durántez A. Lymphokine induction of NK-like cytotoxicity in T cells from B-CLL. Blood. 1986 Jan;67(1):228–232. [PubMed] [Google Scholar]
- Arnaiz-Villena A., Timón M., Rodríguez-Gallego C., Pérez-Blas M., Corell A., Martín-Villa J. M., Regueiro J. R. Human T-cell activation deficiencies. Immunol Today. 1992 Jul;13(7):259–265. doi: 10.1016/0167-5699(92)90007-t. [DOI] [PubMed] [Google Scholar]
- Carmel R., Boone D., Parker J. W. Lymphocyte surface phenotypes in pernicious anemia. Dig Dis Sci. 1987 Aug;32(8):846–850. doi: 10.1007/BF01296707. [DOI] [PubMed] [Google Scholar]
- Gleeson P. A., Toh B. H. Molecular targets in pernicious anaemia. Immunol Today. 1991 Jul;12(7):233–238. doi: 10.1016/0167-5699(91)90036-S. [DOI] [PubMed] [Google Scholar]
- Imamura N., Fujimura K., Kuramoto A. Lymphocyte subpopulations in pernicious anemia. N Engl J Med. 1984 Jul 5;311(1):56–56. doi: 10.1056/nejm198407053110117. [DOI] [PubMed] [Google Scholar]
- Irvine W. J., Cullen D. R., Mawhinney H. Natural history of autoimmune achlorhydric atrophic gastritis. A 1-15-year follow-up study. Lancet. 1974 Aug 31;2(7879):482–485. doi: 10.1016/s0140-6736(74)92013-3. [DOI] [PubMed] [Google Scholar]
- Karlsson F. A., Burman P., Löf L., Mårdh S. Major parietal cell antigen in autoimmune gastritis with pernicious anemia is the acid-producing H+,K+-adenosine triphosphatase of the stomach. J Clin Invest. 1988 Feb;81(2):475–479. doi: 10.1172/JCI113344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaye M. D. Immunological aspects of gastritis and pernicious anaemia. Baillieres Clin Gastroenterol. 1987 Jul;1(3):487–506. doi: 10.1016/0950-3528(87)90044-3. [DOI] [PubMed] [Google Scholar]
- Kätkä K. Immune functions in pernicious anaemia before and during treatment with vitamin B12. Scand J Haematol. 1984 Jan;32(1):76–82. doi: 10.1111/j.1600-0609.1984.tb00680.x. [DOI] [PubMed] [Google Scholar]
- MacCuish A. C., Urbaniak S. J., Goldstone A. H., Irvine W. J. PHA responsiveness and subpopulations of circulating lymphocytes in pernicious anemia. Blood. 1974 Dec;44(6):849–855. [PubMed] [Google Scholar]
- Manzano L., Alvarez-Mon M., Abreu L., Antonio Vargas J., de la Morena E., Corugedo F., Duràntez A. Functional impairment of natural killer cells in active ulcerative colitis: reversion of the defective natural killer activity by interleukin 2. Gut. 1992 Feb;33(2):246–251. doi: 10.1136/gut.33.2.246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soler J., Remacha A., Nieto M., Gimferrer E. Lymphocyte subpopulations in patients with untreated pernicious anaemia. Scand J Haematol. 1985 Sep;35(3):377–377. doi: 10.1111/j.1600-0609.1985.tb01725.x. [DOI] [PubMed] [Google Scholar]
- Stockbrügger R. W., Menon G. G., Beilby J. O., Mason R. R., Cotton P. B. Gastroscopic screening in 80 patients with pernicious anaemia. Gut. 1983 Dec;24(12):1141–1147. doi: 10.1136/gut.24.12.1141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strickland R. G., Mackay I. R. A reappraisal of the nature and significance of chronic atrophic gastritis. Am J Dig Dis. 1973 May;18(5):426–440. doi: 10.1007/BF01071995. [DOI] [PubMed] [Google Scholar]
- Wodzinski M. A., Forrest M. J., Barnett D., Lawrence A. C. Lymphocyte subpopulations in patients with hydroxocobalamin responsive megaloblastic anaemia. J Clin Pathol. 1985 May;38(5):582–584. doi: 10.1136/jcp.38.5.582. [DOI] [PMC free article] [PubMed] [Google Scholar]


