Abstract
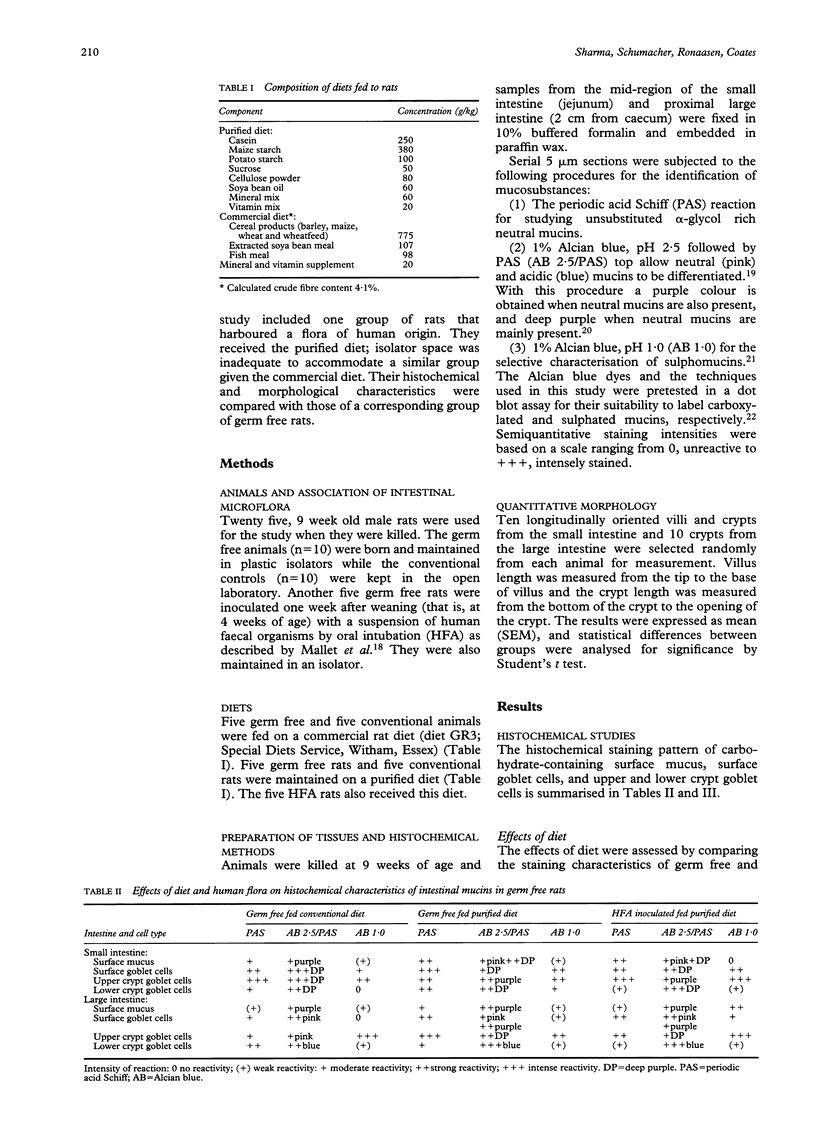
The effects of diet on the histochemical composition of intestinal mucosubstances and the morphology of the villi and crypts were investigated by comparing the data of germ free and conventionally maintained rats fed either a purified diet or a commercial diet. The influence of intestinal microflora was evaluated by comparing the germ free rats and those harbouring either a conventional rat flora or a human microbial flora. In both germ free rats and those maintained conventionally, feeding a purified diet resulted in shallower crypts in the small intestine but deeper crypts in the large intestine compared with their counterparts fed on the commercial diet. The preliminary data obtained with association of human flora showed a reduction of the villus height and crypt depth in the small intestine and, to some extent, the amount of neutral mucins in the goblet cells of both small and large intestine and an increase in the amount of sulphated mucins in the large intestine. In rats given the commercial diet the periodic acid Schiff staining for neutral mucins was more intense in the upper crypts of the small intestine than in the lower crypts, and to a lesser extent in the upper crypts of the large intestine. These results provide evidence that the dietary composition, microbial flora, as well as the interactions between the dietary constituents and microbial flora change the mucosal architecture and the mucus composition and therefore alter the functional characteristics of the intestinal tract.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altmann G. G. Morphological observations on mucus-secreting nongoblet cells in the deep crypts of the rat ascending colon. Am J Anat. 1983 May;167(1):95–117. doi: 10.1002/aja.1001670109. [DOI] [PubMed] [Google Scholar]
- Brackett K. A., Townsend S. F. Organogenesis of the colon in rats. J Morphol. 1980 Feb;163(2):191–201. doi: 10.1002/jmor.1051630207. [DOI] [PubMed] [Google Scholar]
- Cassidy M. M., Lightfoot F. G., Vahouny G. V. Morphological aspects of dietary fibers in the intestine. Adv Lipid Res. 1982;19:203–229. doi: 10.1016/b978-0-12-024919-0.50013-8. [DOI] [PubMed] [Google Scholar]
- Filipe M. I., Fenger C. Histochemical characteristics of mucins in the small intestine. A comparative study of normal mucosa, benign epithelial tumours and carcinoma. Histochem J. 1979 May;11(3):277–287. doi: 10.1007/BF01005027. [DOI] [PubMed] [Google Scholar]
- Filipe M. I. Mucins in the human gastrointestinal epithelium: a review. Invest Cell Pathol. 1979 Jul-Sep;2(3):195–216. [PubMed] [Google Scholar]
- Hill R. R., Cowley H. M., Andremont A. Influence of colonizing micro-flora on the mucin histochemistry of the neonatal mouse colon. Histochem J. 1990 Feb;22(2):102–105. doi: 10.1007/BF01885788. [DOI] [PubMed] [Google Scholar]
- Hoskins L. C., Agustines M., McKee W. B., Boulding E. T., Kriaris M., Niedermeyer G. Mucin degradation in human colon ecosystems. Isolation and properties of fecal strains that degrade ABH blood group antigens and oligosaccharides from mucin glycoproteins. J Clin Invest. 1985 Mar;75(3):944–953. doi: 10.1172/JCI111795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishikawa K., Satoh Y., Tanaka H., Ono K. Influence of conventionalization on small-intestinal mucosa of germ-free Wistar rats: quantitative light microscopic observations. Acta Anat (Basel) 1986;127(4):296–302. doi: 10.1159/000146301. [DOI] [PubMed] [Google Scholar]
- LEV R., SPICER S. S. SPECIFIC STAINING OF SULPHATE GROUPS WITH ALCIAN BLUE AT LOW PH. J Histochem Cytochem. 1964 Apr;12:309–309. doi: 10.1177/12.4.309. [DOI] [PubMed] [Google Scholar]
- Mallett A. K., Bearne C. A., Rowland I. R., Farthing M. J., Cole C. B., Fuller R. The use of rats associated with a human faecal flora as a model for studying the effects of diet on the human gut microflora. J Appl Bacteriol. 1987 Jul;63(1):39–45. doi: 10.1111/j.1365-2672.1987.tb02415.x. [DOI] [PubMed] [Google Scholar]
- Moré J., Fioramonti J., Bénazet F., Buéno L. Histochemical characterization of glycoproteins present in jejunal and colonic goblet cells of pigs on different diets. A biopsy study using chemical methods and peroxidase-labelled lectins. Histochemistry. 1987;87(2):189–194. doi: 10.1007/BF00533405. [DOI] [PubMed] [Google Scholar]
- Park C. M., Reid P. E., Owen D. A., Volz D., Dunn W. L. Histochemical studies of epithelial cell glycoproteins in normal rat colon. Histochem J. 1987 Oct-Nov;19(10-11):546–554. doi: 10.1007/BF01687362. [DOI] [PubMed] [Google Scholar]
- Ravdin J. I., John J. E., Johnston L. I., Innes D. J., Guerrant R. L. Adherence of Entamoeba histolytica trophozoites to rat and human colonic mucosa. Infect Immun. 1985 May;48(2):292–297. doi: 10.1128/iai.48.2.292-297.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid P. E., Culling C. F., Dunn W. L. The histochemical interpretation of the complex results of methylation upon gastrointestinal tract mucins, with special reference to the periodic acid-Schiff reactivity. J Histochem Cytochem. 1974 Oct;22(10):986–991. doi: 10.1177/22.10.986. [DOI] [PubMed] [Google Scholar]
- Rozee K. R., Cooper D., Lam K., Costerton J. W. Microbial flora of the mouse ileum mucous layer and epithelial surface. Appl Environ Microbiol. 1982 Jun;43(6):1451–1463. doi: 10.1128/aem.43.6.1451-1463.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rumney C. J., Rowland I. R. In vivo and in vitro models of the human colonic flora. Crit Rev Food Sci Nutr. 1992;31(4):299–331. doi: 10.1080/10408399209527575. [DOI] [PubMed] [Google Scholar]
- SPICER S. S. A correlative study of the histochemical properties of rodent acid mucopolysaccharides. J Histochem Cytochem. 1960 Jan;8:18–35. doi: 10.1177/8.1.18. [DOI] [PubMed] [Google Scholar]
- SPICER S. S. DIAMINE METHODS FOR DIFFERENTIALING MUCOSUBSTANCES HISTOCHEMICALLY. J Histochem Cytochem. 1965 Mar;13:211–234. doi: 10.1177/13.3.211. [DOI] [PubMed] [Google Scholar]
- Savage D. C. Factors involved in colonization of the gut epithelial surface. Am J Clin Nutr. 1978 Oct;31(10 Suppl):S131–S135. doi: 10.1093/ajcn/31.10.S131. [DOI] [PubMed] [Google Scholar]
- Schumacher U., Adam E. Standardization of staining in glycosaminoglycan histochemistry: alcian blue, its analogues, and diamine methods. Biotech Histochem. 1994 Jan;69(1):18–24. doi: 10.3109/10520299409106256. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A. K., Trump B. F. Colon epithelium. I. Light microscopic, histochemical, and ultrastructural features of normal colon epithelium of male Fischer 344 rats. J Natl Cancer Inst. 1981 Feb;66(2):375–388. [PubMed] [Google Scholar]
- Sharma R., Schumacher U. Histochemical characterization of carbohydrate residues during the morphogenesis of gastrointestinal and respiratory systems of Caretta caretta. Acta Histochem. 1992;93(2):411–432. doi: 10.1016/S0065-1281(11)80111-X. [DOI] [PubMed] [Google Scholar]
- Sheahan D. G., Jervis H. R. Comparative histochemistry of gastrointestinal mucosubstances. Am J Anat. 1976 Jun;146(2):103–131. doi: 10.1002/aja.1001460202. [DOI] [PubMed] [Google Scholar]
- Silberberg A., Meyer F. A. Structure and function of mucus. Adv Exp Med Biol. 1982;144:53–74. doi: 10.1007/978-1-4615-9254-9_6. [DOI] [PubMed] [Google Scholar]
- Tasman-Jones C., Owen R. L., Jones A. L. Semipurified dietary fiber and small-bowel morphology in rats. Dig Dis Sci. 1982 Jun;27(6):519–524. doi: 10.1007/BF01296731. [DOI] [PubMed] [Google Scholar]
- Thomopoulos G. N., Schulte B. A., Spicer S. S. Light and electron microscopic cytochemistry of glycoconjugates in the rectosigmoid colonic epithelium of the mouse and rat. Am J Anat. 1983 Oct;168(2):239–256. doi: 10.1002/aja.1001680210. [DOI] [PubMed] [Google Scholar]
- Vahouny G. V., Le T., Ifrim I., Satchithanandam S., Cassidy M. M. Stimulation of intestinal cytokinetics and mucin turnover in rats fed wheat bran or cellulose. Am J Clin Nutr. 1985 May;41(5):895–900. doi: 10.1093/ajcn/41.5.895. [DOI] [PubMed] [Google Scholar]
- Vercellotti J. R., Salyers A. A., Bullard W. S., Wilkins D. Breakdown of mucin and plant polysaccharides in the human colon. Can J Biochem. 1977 Nov;55(11):1190–1196. doi: 10.1139/o77-178. [DOI] [PubMed] [Google Scholar]




