Abstract
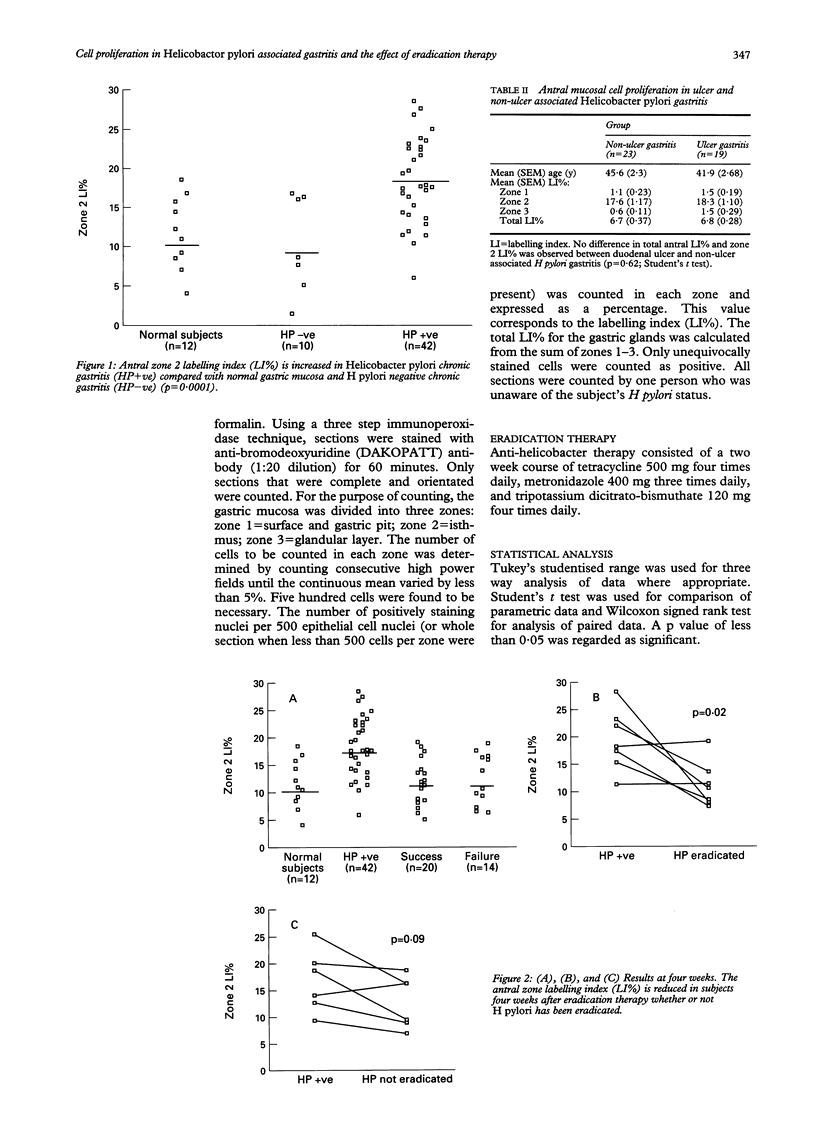
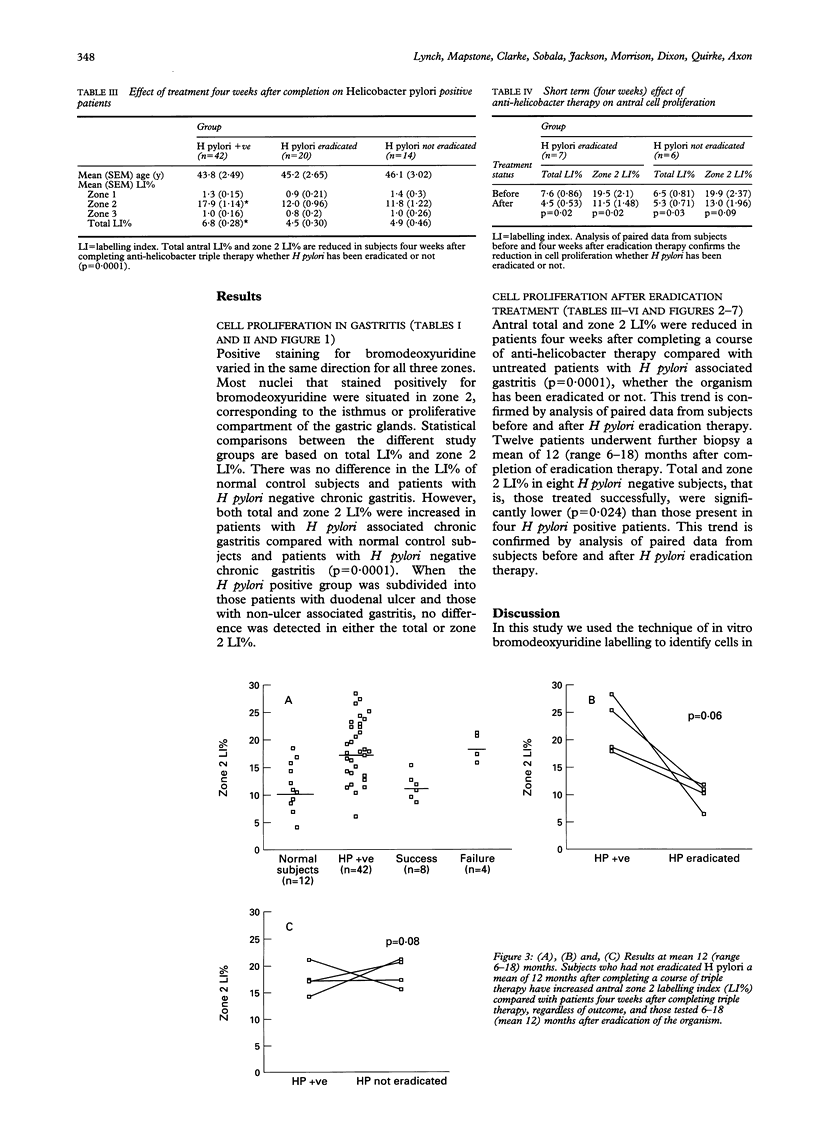
Helicobacter pylori causes chronic (type B) gastritis. The 'intestinal' form of gastric cancer arises against a background of chronic gastritis, and prospective epidemiological studies have shown that H pylori is a major risk factor for this. An increase in mucosal cell proliferation increases the likelihood of a neoplastic clone of epithelial cells emerging where there is chronic epithelial cell injury associated with H pylori gastritis. In vitro bromodeoxyuridine labelling of endoscopic antral biopsy specimens was used to measure mucosal cell proliferation in H pylori associated gastritis before and after therapy for H pylori triple infection. Cell proliferation was increased in H pylori associated gastritis patients compared with normal controls and patients with H pylori negative chronic gastritis (p = 0.0001; Tukey's Studentised range). There was no difference in antral epithelial cell proliferation between duodenal ulcer and non-ulcer subjects infected with H pylori (p = 0.62; Student's t test). Antral mucosal cell proliferation fell four weeks after completing triple therapy, irrespective of whether or not H pylori had been eradicated (p = 0.0001). At retesting six to 18 months later (mean = 12 months), however, those in whom H pylori had not been successfully eradicated showed increased mucosal proliferation compared with both H pylori negative subjects at a similar follow up interval and all cases (whether H pylori positive or negative) four weeks after completion of triple therapy (p = 0.024). These findings suggest that H pylori infection causes increased gastric cell proliferation and in this way may play a part in gastric carcinogenesis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ames B. N., Gold L. S. Too many rodent carcinogens: mitogenesis increases mutagenesis. Science. 1990 Aug 31;249(4972):970–971. doi: 10.1126/science.2136249. [DOI] [PubMed] [Google Scholar]
- Dixon M. F. Helicobacter pylori and peptic ulceration: histopathological aspects. J Gastroenterol Hepatol. 1991 Mar-Apr;6(2):125–130. doi: 10.1111/j.1440-1746.1991.tb01451.x. [DOI] [PubMed] [Google Scholar]
- Emery P., Salmon M. The immune response. 2. Systemic mediators of inflammation. Br J Hosp Med. 1991 Mar;45(3):164–168. [PubMed] [Google Scholar]
- Forman D., Newell D. G., Fullerton F., Yarnell J. W., Stacey A. R., Wald N., Sitas F. Association between infection with Helicobacter pylori and risk of gastric cancer: evidence from a prospective investigation. BMJ. 1991 Jun 1;302(6788):1302–1305. doi: 10.1136/bmj.302.6788.1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hall P. A., Lemoine N. R. Rapid acinar to ductal transdifferentiation in cultured human exocrine pancreas. J Pathol. 1992 Feb;166(2):97–103. doi: 10.1002/path.1711660203. [DOI] [PubMed] [Google Scholar]
- Hart-Hansen O., Johansen A., Larsen J. K., Svendsen L. B. Cell proliferation in normal and diseased gastric mucosa. Autoradiography after in vitro continuous labelling with tritiated thymidine. Acta Pathol Microbiol Scand A. 1979 May;87A(3):217–222. [PubMed] [Google Scholar]
- Jankowski J., al-Rawi H. J., Johnston D. A., Hopwood D., Filipe M. I., Coghill G., Wormsley K. G. Growth regulatory peptides in gastric mucosa. Clin Sci (Lond) 1992 May;82(5):581–587. doi: 10.1042/cs0820581. [DOI] [PubMed] [Google Scholar]
- Lacy E. R., Kuwayama H., Cowart K. S., King J. S., Deutz A. H., Sistrunk S. A rapid, accurate, immunohistochemical method to label proliferating cells in the digestive tract. A comparison with tritiated thymidine. Gastroenterology. 1991 Jan;100(1):259–262. doi: 10.1016/0016-5085(91)90610-w. [DOI] [PubMed] [Google Scholar]
- Lunec J. Free radicals: their involvement in disease processes. Ann Clin Biochem. 1990 May;27(Pt 3):173–182. doi: 10.1177/000456329002700301. [DOI] [PubMed] [Google Scholar]
- Lynch D. A., Clarke A. M., Jackson P., Axon A. T., Dixon M. F., Quirke P. Comparison of labelling by bromodeoxyuridine, MIB-1, and proliferating cell nuclear antigen in gastric mucosal biopsy specimens. J Clin Pathol. 1994 Feb;47(2):122–125. doi: 10.1136/jcp.47.2.122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MORSON B. C. Intestinal metaplasia of the gastric mucosa. Br J Cancer. 1955 Sep;9(3):365–376. doi: 10.1038/bjc.1955.35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mackerness C. W., Leach S. A., Thompson M. H., Hill M. J. The inhibition of bacterially mediated N-nitrosation by vitamin C: relevance to the inhibition of endogenous N-nitrosation in the achlorhydric stomach. Carcinogenesis. 1989 Feb;10(2):397–399. doi: 10.1093/carcin/10.2.397. [DOI] [PubMed] [Google Scholar]
- Marshall B. J. The use of bismuth in gastroenterology. The ACG Committee on FDA-Related Matters. American College of Gastroenterology. Am J Gastroenterol. 1991 Jan;86(1):16–25. [PubMed] [Google Scholar]
- Mirvish S. S., Wallcave L., Eagen M., Shubik P. Ascorbate-nitrite reaction: possible means of blocking the formation of carcinogenic N-nitroso compounds. Science. 1972 Jul 7;177(4043):65–68. doi: 10.1126/science.177.4043.65. [DOI] [PubMed] [Google Scholar]
- Nomura A., Stemmermann G. N., Chyou P. H., Kato I., Perez-Perez G. I., Blaser M. J. Helicobacter pylori infection and gastric carcinoma among Japanese Americans in Hawaii. N Engl J Med. 1991 Oct 17;325(16):1132–1136. doi: 10.1056/NEJM199110173251604. [DOI] [PubMed] [Google Scholar]
- Ohyama S., Yonemura Y., Miyazaki I. Proliferative activity and malignancy in human gastric cancers. Significance of the proliferation rate and its clinical application. Cancer. 1992 Jan 15;69(2):314–321. doi: 10.1002/1097-0142(19920115)69:2<314::aid-cncr2820690207>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- Parsonnet J., Friedman G. D., Vandersteen D. P., Chang Y., Vogelman J. H., Orentreich N., Sibley R. K. Helicobacter pylori infection and the risk of gastric carcinoma. N Engl J Med. 1991 Oct 17;325(16):1127–1131. doi: 10.1056/NEJM199110173251603. [DOI] [PubMed] [Google Scholar]
- Rathbone B. J., Johnson A. W., Wyatt J. I., Kelleher J., Heatley R. V., Losowsky M. S. Ascorbic acid: a factor concentrated in human gastric juice. Clin Sci (Lond) 1989 Mar;76(3):237–241. doi: 10.1042/cs0760237. [DOI] [PubMed] [Google Scholar]
- Rauws E. A., Langenberg W., Houthoff H. J., Zanen H. C., Tytgat G. N. Campylobacter pyloridis-associated chronic active antral gastritis. A prospective study of its prevalence and the effects of antibacterial and antiulcer treatment. Gastroenterology. 1988 Jan;94(1):33–40. [PubMed] [Google Scholar]
- Sobala G. M., Schorah C. J., Sanderson M., Dixon M. F., Tompkins D. S., Godwin P., Axon A. T. Ascorbic acid in the human stomach. Gastroenterology. 1989 Aug;97(2):357–363. doi: 10.1016/0016-5085(89)90071-1. [DOI] [PubMed] [Google Scholar]
- Sobala G. M., Schorah C. J., Shires S., Lynch D. A., Gallacher B., Dixon M. F., Axon A. T. Effect of eradication of Helicobacter pylori on gastric juice ascorbic acid concentrations. Gut. 1993 Aug;34(8):1038–1041. doi: 10.1136/gut.34.8.1038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wijsman J. H., Van Dierendonck J. H., Keijzer R., van de Velde C. J., Cornelisse C. J. Immunoreactivity of proliferating cell nuclear antigen compared with bromodeoxyuridine incorporation in normal and neoplastic rat tissue. J Pathol. 1992 Sep;168(1):75–83. doi: 10.1002/path.1711680113. [DOI] [PubMed] [Google Scholar]


