Abstract
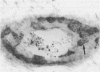
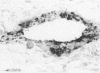
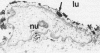
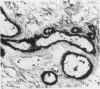
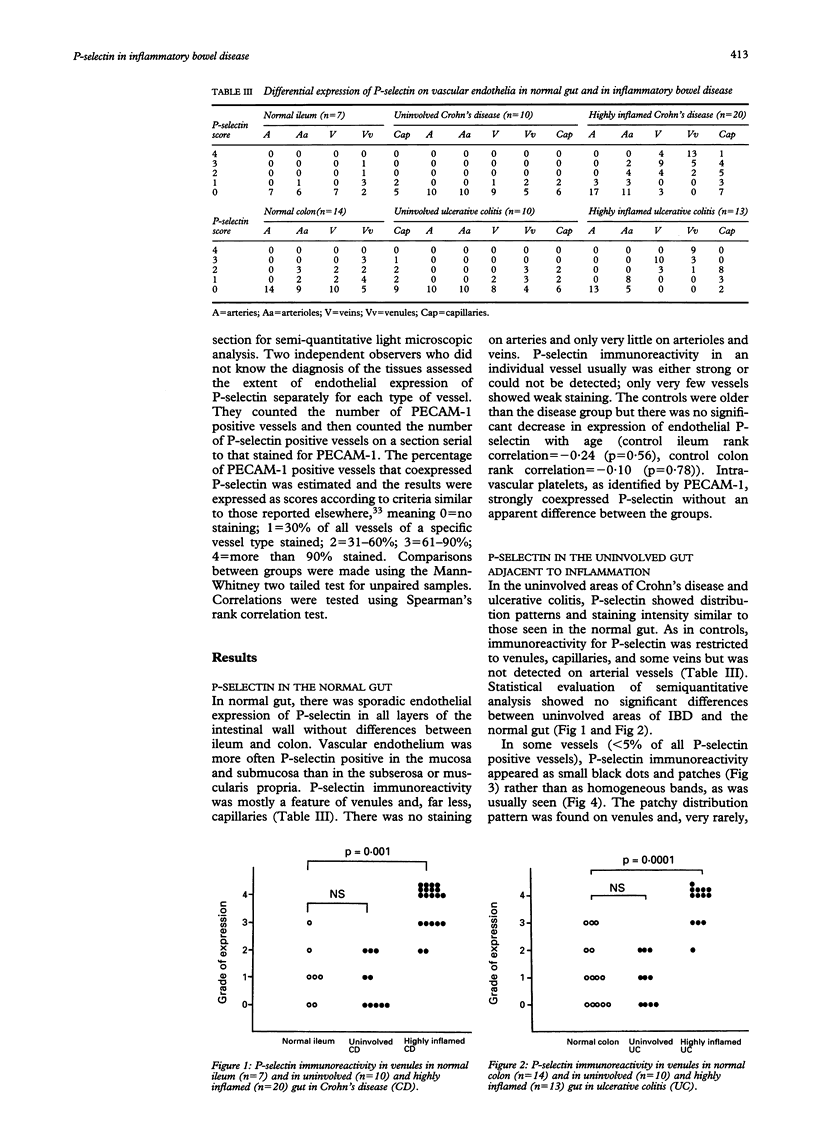
The pathogenic changes of inflammatory bowel disease (IBD) depend on migration of circulating leucocytes into intestinal tissues. Although leucocyte rolling and tenuous adhesion are probably regulated by inducible selectins on vascular endothelia, little is known about the expression of these molecules in Crohn's disease and ulcerative colitis. Using immunohistochemistry on surgically resected specimens, this study investigated endothelial P-selectin (CD62, granular membrane protein-140) in frozen sections of histologically uninvolved tissues adjacent to inflammation (Crohn's disease = 10; ulcerative colitis = 10), from highly inflamed areas (Crohn's disease = 20; ulcerative colitis = 13), and from normal bowel (n = 20). By light microscopy, two forms of P-selectin immunoreactivity were detected that apparently corresponded ultrastructurally to stored and released distributions. Compared with the normal gut, there was a 3.7-fold increase of P-selectin immunoreactivity on veins (p < 0.0001), venules (p < 0.0001), and capillaries (p < 0.05) in the highly inflamed gut, without differences between Crohn's disease and ulcerative colitis. In the uninvolved gut, P-selectin expression was similar to that seen in normal controls, except for a focal increase of P-selectin in the vicinity of small lymphocyte aggregates. The dramatic upregulation of P-selectin in the inflamed tissue and its potential role in leucocyte trafficking support the concept of P-selectin blocking therapy for the control of active IBD.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bishop A. E., Polak J. M., Bryant M. G., Bloom S. R., Hamilton S. Abnormalities of vasoactive intestinal polypeptide-containing nerves in Crohn's disease. Gastroenterology. 1980 Nov;79(5 Pt 1):853–860. [PubMed] [Google Scholar]
- Carden D. L., Young J. A., Granger D. N. Pulmonary microvascular injury after intestinal ischemia-reperfusion: role of P-selectin. J Appl Physiol (1985) 1993 Dec;75(6):2529–2534. doi: 10.1152/jappl.1993.75.6.2529. [DOI] [PubMed] [Google Scholar]
- Collins C. E., Cahill M. R., Newland A. C., Rampton D. S. Platelets circulate in an activated state in inflammatory bowel disease. Gastroenterology. 1994 Apr;106(4):840–845. doi: 10.1016/0016-5085(94)90741-2. [DOI] [PubMed] [Google Scholar]
- Collins P. W., Macey M. G., Cahill M. R., Newland A. C. von Willebrand factor release and P-selectin expression is stimulated by thrombin and trypsin but not IL-1 in cultured human endothelial cells. Thromb Haemost. 1993 Aug 2;70(2):346–350. [PubMed] [Google Scholar]
- Coughlan A. F., Hau H., Dunlop L. C., Berndt M. C., Hancock W. W. P-selectin and platelet-activating factor mediate initial endotoxin-induced neutropenia. J Exp Med. 1994 Jan 1;179(1):329–334. doi: 10.1084/jem.179.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damle N. K., Klussman K., Dietsch M. T., Mohagheghpour N., Aruffo A. GMP-140 (P-selectin/CD62) binds to chronically stimulated but not resting CD4+ T lymphocytes and regulates their production of proinflammatory cytokines. Eur J Immunol. 1992 Jul;22(7):1789–1793. doi: 10.1002/eji.1830220718. [DOI] [PubMed] [Google Scholar]
- Dustin M. L., Springer T. A. Role of lymphocyte adhesion receptors in transient interactions and cell locomotion. Annu Rev Immunol. 1991;9:27–66. doi: 10.1146/annurev.iy.09.040191.000331. [DOI] [PubMed] [Google Scholar]
- Erbe D. V., Watson S. R., Presta L. G., Wolitzky B. A., Foxall C., Brandley B. K., Lasky L. A. P- and E-selectin use common sites for carbohydrate ligand recognition and cell adhesion. J Cell Biol. 1993 Mar;120(5):1227–1235. doi: 10.1083/jcb.120.5.1227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etingin O. R., Silverstein R. L., Hajjar D. P. Identification of a monocyte receptor on herpesvirus-infected endothelial cells. Proc Natl Acad Sci U S A. 1991 Aug 15;88(16):7200–7203. doi: 10.1073/pnas.88.16.7200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferran C., Peuchmaur M., Desruennes M., Ghoussoub J. J., Cabrol A., Brousse N., Cabrol C., Bach J. F., Chatenoud L. Implications of de novo ELAM-1 and VCAM-1 expression in human cardiac allograft rejection. Transplantation. 1993 Mar;55(3):605–609. doi: 10.1097/00007890-199303000-00026. [DOI] [PubMed] [Google Scholar]
- Geboes K., Rutgeerts P., Ectors N., Mebis J., Penninckx F., Vantrappen G., Desmet V. J. Major histocompatibility class II expression on the small intestinal nervous system in Crohn's disease. Gastroenterology. 1992 Aug;103(2):439–447. doi: 10.1016/0016-5085(92)90832-j. [DOI] [PubMed] [Google Scholar]
- Hattori R., Hamilton K. K., Fugate R. D., McEver R. P., Sims P. J. Stimulated secretion of endothelial von Willebrand factor is accompanied by rapid redistribution to the cell surface of the intracellular granule membrane protein GMP-140. J Biol Chem. 1989 May 15;264(14):7768–7771. [PubMed] [Google Scholar]
- Hogg N. Roll, roll, roll your leucocyte gently down the vein.... Immunol Today. 1992 Apr;13(4):113–115. doi: 10.1016/0167-5699(92)90103-E. [DOI] [PubMed] [Google Scholar]
- Horak E. R., Leek R., Klenk N., LeJeune S., Smith K., Stuart N., Greenall M., Stepniewska K., Harris A. L. Angiogenesis, assessed by platelet/endothelial cell adhesion molecule antibodies, as indicator of node metastases and survival in breast cancer. Lancet. 1992 Nov 7;340(8828):1120–1124. doi: 10.1016/0140-6736(92)93150-l. [DOI] [PubMed] [Google Scholar]
- Hsu S. M., Raine L., Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981 Apr;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Johnston G. I., Cook R. G., McEver R. P. Cloning of GMP-140, a granule membrane protein of platelets and endothelium: sequence similarity to proteins involved in cell adhesion and inflammation. Cell. 1989 Mar 24;56(6):1033–1044. doi: 10.1016/0092-8674(89)90636-3. [DOI] [PubMed] [Google Scholar]
- Koizumi M., King N., Lobb R., Benjamin C., Podolsky D. K. Expression of vascular adhesion molecules in inflammatory bowel disease. Gastroenterology. 1992 Sep;103(3):840–847. doi: 10.1016/0016-5085(92)90015-q. [DOI] [PubMed] [Google Scholar]
- Koretz K., Momburg F., Otto H. F., Möller P. Sequential induction of MHC antigens on autochthonous cells of ileum affected by Crohn's disease. Am J Pathol. 1987 Dec;129(3):493–502. [PMC free article] [PubMed] [Google Scholar]
- Lawrence M. B., Springer T. A. Leukocytes roll on a selectin at physiologic flow rates: distinction from and prerequisite for adhesion through integrins. Cell. 1991 May 31;65(5):859–873. doi: 10.1016/0092-8674(91)90393-d. [DOI] [PubMed] [Google Scholar]
- Lorant D. E., Topham M. K., Whatley R. E., McEver R. P., McIntyre T. M., Prescott S. M., Zimmerman G. A. Inflammatory roles of P-selectin. J Clin Invest. 1993 Aug;92(2):559–570. doi: 10.1172/JCI116623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malizia G., Calabrese A., Cottone M., Raimondo M., Trejdosiewicz L. K., Smart C. J., Oliva L., Pagliaro L. Expression of leukocyte adhesion molecules by mucosal mononuclear phagocytes in inflammatory bowel disease. Gastroenterology. 1991 Jan;100(1):150–159. doi: 10.1016/0016-5085(91)90595-c. [DOI] [PubMed] [Google Scholar]
- McEver R. P., Beckstead J. H., Moore K. L., Marshall-Carlson L., Bainton D. F. GMP-140, a platelet alpha-granule membrane protein, is also synthesized by vascular endothelial cells and is localized in Weibel-Palade bodies. J Clin Invest. 1989 Jul;84(1):92–99. doi: 10.1172/JCI114175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore K. L., Thompson L. F. P-selectin (CD62) binds to subpopulations of human memory T lymphocytes and natural killer cells. Biochem Biophys Res Commun. 1992 Jul 15;186(1):173–181. doi: 10.1016/s0006-291x(05)80790-9. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Paulson J. C., De Frees S., Zheng Z. L., Lowe J. B., Ward P. A. Protective effects of oligosaccharides in P-selectin-dependent lung injury. Nature. 1993 Jul 8;364(6433):149–151. doi: 10.1038/364149a0. [DOI] [PubMed] [Google Scholar]
- Mulligan M. S., Polley M. J., Bayer R. J., Nunn M. F., Paulson J. C., Ward P. A. Neutrophil-dependent acute lung injury. Requirement for P-selectin (GMP-140). J Clin Invest. 1992 Oct;90(4):1600–1607. doi: 10.1172/JCI116029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murch S. H., Braegger C. P., Walker-Smith J. A., MacDonald T. T. Location of tumour necrosis factor alpha by immunohistochemistry in chronic inflammatory bowel disease. Gut. 1993 Dec;34(12):1705–1709. doi: 10.1136/gut.34.12.1705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura S., Ohtani H., Watanabe Y., Fukushima K., Matsumoto T., Kitano A., Kobayashi K., Nagura H. In situ expression of the cell adhesion molecules in inflammatory bowel disease. Evidence of immunologic activation of vascular endothelial cells. Lab Invest. 1993 Jul;69(1):77–85. [PubMed] [Google Scholar]
- Ohtani H., Nakamura S., Watanabe Y., Fukushima K., Mizoi T., Kimura M., Hiwatashi N., Nagura H. Light and electron microscopic immunolocalization of endothelial leucocyte adhesion molecule-1 in inflammatory bowel disease. Morphological evidence of active synthesis and secretion into vascular lumen. Virchows Arch A Pathol Anat Histopathol. 1992;420(5):403–409. doi: 10.1007/BF01600511. [DOI] [PubMed] [Google Scholar]
- Patel K. D., Zimmerman G. A., Prescott S. M., McEver R. P., McIntyre T. M. Oxygen radicals induce human endothelial cells to express GMP-140 and bind neutrophils. J Cell Biol. 1991 Feb;112(4):749–759. doi: 10.1083/jcb.112.4.749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picker L. J., Warnock R. A., Burns A. R., Doerschuk C. M., Berg E. L., Butcher E. C. The neutrophil selectin LECAM-1 presents carbohydrate ligands to the vascular selectins ELAM-1 and GMP-140. Cell. 1991 Sep 6;66(5):921–933. doi: 10.1016/0092-8674(91)90438-5. [DOI] [PubMed] [Google Scholar]
- Polley M. J., Phillips M. L., Wayner E., Nudelman E., Singhal A. K., Hakomori S., Paulson J. C. CD62 and endothelial cell-leukocyte adhesion molecule 1 (ELAM-1) recognize the same carbohydrate ligand, sialyl-Lewis x. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6224–6228. doi: 10.1073/pnas.88.14.6224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rampton D. S., Murdoch R. D., Sladen G. E. Rectal mucosal histamine release in ulcerative colitis. Clin Sci (Lond) 1980 Nov;59(5):389–391. doi: 10.1042/cs0590389. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Camilleri M., Rees H., Lavender J. P., Hodgson H. J., Chadwick V. S. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986 May;90(5 Pt 1):1121–1128. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- Schuermann G. M., Aber-Bishop A. E., Facer P., Lee J. C., Rampton D. S., Doré C. J., Polak J. M. Altered expression of cell adhesion molecules in uninvolved gut in inflammatory bowel disease. Clin Exp Immunol. 1993 Nov;94(2):341–347. doi: 10.1111/j.1365-2249.1993.tb03455.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimizu Y., Newman W., Tanaka Y., Shaw S. Lymphocyte interactions with endothelial cells. Immunol Today. 1992 Mar;13(3):106–112. doi: 10.1016/0167-5699(92)90151-V. [DOI] [PubMed] [Google Scholar]
- Simmonds N. J., Allen R. E., Stevens T. R., Van Someren R. N., Blake D. R., Rampton D. S. Chemiluminescence assay of mucosal reactive oxygen metabolites in inflammatory bowel disease. Gastroenterology. 1992 Jul;103(1):186–196. doi: 10.1016/0016-5085(92)91112-h. [DOI] [PubMed] [Google Scholar]
- Steinhoff G., Behrend M., Schrader B., Duijvestijn A. M., Wonigeit K. Expression patterns of leukocyte adhesion ligand molecules on human liver endothelia. Lack of ELAM-1 and CD62 inducibility on sinusoidal endothelia and distinct distribution of VCAM-1, ICAM-1, ICAM-2, and LFA-3. Am J Pathol. 1993 Feb;142(2):481–488. [PMC free article] [PubMed] [Google Scholar]
- Taylor P. M., Rose M. L., Yacoub M. H., Pigott R. Induction of vascular adhesion molecules during rejection of human cardiac allografts. Transplantation. 1992 Sep;54(3):451–457. doi: 10.1097/00007890-199209000-00013. [DOI] [PubMed] [Google Scholar]
- Ushiyama S., Laue T. M., Moore K. L., Erickson H. P., McEver R. P. Structural and functional characterization of monomeric soluble P-selectin and comparison with membrane P-selectin. J Biol Chem. 1993 Jul 15;268(20):15229–15237. [PubMed] [Google Scholar]
- Wakefield A. J., Pittilo R. M., Sim R., Cosby S. L., Stephenson J. R., Dhillon A. P., Pounder R. E. Evidence of persistent measles virus infection in Crohn's disease. J Med Virol. 1993 Apr;39(4):345–353. doi: 10.1002/jmv.1890390415. [DOI] [PubMed] [Google Scholar]
- Weller A., Isenmann S., Vestweber D. Cloning of the mouse endothelial selectins. Expression of both E- and P-selectin is inducible by tumor necrosis factor alpha. J Biol Chem. 1992 Jul 25;267(21):15176–15183. [PubMed] [Google Scholar]
- Weyrich A. S., Ma X. Y., Lefer D. J., Albertine K. H., Lefer A. M. In vivo neutralization of P-selectin protects feline heart and endothelium in myocardial ischemia and reperfusion injury. J Clin Invest. 1993 Jun;91(6):2620–2629. doi: 10.1172/JCI116501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiedenmann B., Waldherr R., Buhr H., Hille A., Rosa P., Huttner W. B. Identification of gastroenteropancreatic neuroendocrine cells in normal and neoplastic human tissue with antibodies against synaptophysin, chromogranin A, secretogranin I (chromogranin B), and secretogranin II. Gastroenterology. 1988 Nov;95(5):1364–1374. doi: 10.1016/0016-5085(88)90374-5. [DOI] [PubMed] [Google Scholar]
- Winn R. K., Harlan J. M. CD18-independent neutrophil and mononuclear leukocyte emigration into the peritoneum of rabbits. J Clin Invest. 1993 Sep;92(3):1168–1173. doi: 10.1172/JCI116686. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamazaki T., Seko Y., Tamatani T., Miyasaka M., Yagita H., Okumura K., Nagai R., Yazaki Y. Expression of intercellular adhesion molecule-1 in rat heart with ischemia/reperfusion and limitation of infarct size by treatment with antibodies against cell adhesion molecules. Am J Pathol. 1993 Aug;143(2):410–418. [PMC free article] [PubMed] [Google Scholar]