Abstract
The objective of this study was to examine the independent effect of infection with each of four helminths (Ascaris lumbricoides, Schistosoma japonicum, Necator americanus, and Trichuris trichiura) on cognitive function after adjusting for the potential confounders nutritional status, socioeconomic status (SES), hemoglobin, sex, and the presence of other helminthes. This cross-sectional study was carried out in a rural village in Leyte, The Philippines in 319 children 7–18 years old. Three stools were collected and read in duplicate by the Kato Katz method. Infection intensity was defined by World Health Organization criteria. Cognitive tests were culturally adapted and translated. Learning and memory cognitive domains were each defined by three subscales of the Wide Range Assessment of Memory and Learning, which had an inter-rater reliability ≥ 0.92 and test-retest reliabilities ranging from 0.61 to 0.89. A household SES questionnaire was administered. A logistic regression model was used to quantify the association between performance in different cognitive domains (learning, memory, verbal fluency, and the Philippine Non-Verbal Intelligence Test) and helminth infections. After adjusting for age, sex, nutritional status, hemoglobin, and SES, S. japonicum infection was associated with poor performance on tests of learning (odds ratio [OR] = 3.04, 95% confidence interval [CI] = 1.1–6.9), A. lumbricoides infection was associated with poor performance on tests of memory (OR = 2.2, 95% CI = 1.04–4.7), and T. trichiura infection was associated with poor performance on tests of verbal fluency (OR = 4.5, 95% CI = 1.04–30). Helminth infection was associated with lower performance in three of the four cognitive domains examined in this study. These relationships remained after rigorous control for other helminths and important confounding covariates.
INTRODUCTION
Helminth infections are important causes of morbidity and mortality in many developing countries. An estimated 1,471 million cases of infection with Ascaris lumbricoides, 1,200 million cases of infection with hookworm, 1,049 million cases of infection with Trichuris trichiura, and 200–300 million cases of schistosomiasis occur worldwide.1-4 School age children in developing countries bear the greatest health burden due to helminth infections.5,6 According to a World Bank report, morbidity due to helminth infections accounts for an estimated 20% of the disability-adjusted life years lost due to infectious diseases in children less than 14 years old.7 Among the well-described morbidities associated with helminth infection in children are under-nutrition, anemia, and failure to achieve genetic potential for growth.2,8-12
Several studies have examined the association between helminth infection and children's academic performance or cognitive function.13 Results and conclusions across studies have been inconsistent and often conflicting. Some of the observed inconsistencies may be related to study design flaws that include failure to adapt cognitive tests to study populations and inadequate control for important confounding variables such as socioeconomic status (SES).13-15 The specific helminth infection and its intensity may also contribute to variability in findings. Despite this, most investigators agree that helminth control initiatives are beneficial to children's cognitive performance based on results from well-designed studies. For example, one randomized controlled trial conducted in China16 demonstrated improvement in working memory following treatment of Schistosoma japonicum infection among primary school children. One observational study among Tanzanian children found that high intensity infection with S. haematobium is associated with poor short-term memory.17 Biovin and others also found improvements in cognitive performance among Zairian children after treating for intestinal helminths, particularly with treatment of hookworm infection.18-20 Similar positive associations between cognitive performance and treatment of infection with Trichuris and Ascaris have also been reported.2,14
Limitations exist in the current literature. Many of the aforementioned studies are randomized controlled trials. These studies, despite having the advantage of allowing causal inferences to be made in a way that cross-sectional studies cannot, have some drawbacks. Short periods of follow-up, as is often the case in many controlled trials, may miss effects of treatment because they do not allow sufficient time to observe outcome improvements. In addition, well-designed cross-sectional studies that control for important potential confounders may be better able to integrate the long-standing, cumulative effects of helminth infections on cognitive processes, despite their weaknesses for causal inference.21 Furthermore, studies to date have focused on memory and attention, with little focus on learning ability. The learning domain of cognitive function may better reflect a child's ability to take advantage of the limited educational opportunities available in the developing world. Finally, the mechanisms underlying the relationship between helminth infection and cognitive processes have not been examined.
This study was conducted in the context of a longitudinal treatment-reinfection study assessing the relationship between S. japonicum infection and cognitive performance. Baseline cross-sectional results are presented. The objectives of this study were to 1) assess the relationship between S. japonicum infection and cognitive function, with an emphasis on learning ability after controlling for important potential confounders, and 2) explore the mechanistic role of hemoglobin and under-nutrition as mediators of decreased cognitive function in the context of helminth infection.
METHODS
Study population. This study was conducted in a rural rice-farming village in Leyte, The Philippines, an area endemic for both S. japonicum and intestinal helminthes.22,23 Three hundred thirty-one school age children (185 boys and 146 girls) between the ages of 7 and 18 years were enrolled into the study. Twelve subjects had missing/incomplete data with respect to parasite infection status (n = 2), cognitive test scores (n = 6), and nutritional status (n = 4), leaving an effective study sample of 319 children. All subjects were recruited from a single village, where the main study was conducted. Subjects were eligible if they provided a stool sample, child assent, and parental consent. Exclusion criteria included failure to submit a stool sample, current pregnancy, and history or physical examination findings consistent with presence of a serious chronic disease, e.g., active tuberculosis or developmental disability. This study was reviewed and approved by the Brown University and Philippines Research Institute of Tropical Medicine Institutional Review Boards.
Infection intensity. Parasite burden was determined by examination of three stool specimens from each study participant. Each of the three specimens were examined in duplicate for S. japonicum, hookworm, T. trichiura, and A. lumbricoides by the Kato Katz method. Intensity of infection for each parasite was defined by number of eggs per gram (epg) of feces using the World Health Organization criteria for low-, moderate-, or high-intensity infection for S. japonicum, A. lumbricoides, T. trichiura, and hookworm. For each of the stool specimens, the average epg of the duplicate tests was used; intensity of infection for each parasite was obtained by averaging the epg of the three fecal specimens. For S. japonicum, low-, medium-, and high-intensity infection categories were defined as 1–99 epg, 100–399 epg, and ≥ 400 epg, respectively. For T. trichiura and hookworm, the low-intensity infection category was defined as 1–999 epg and the moderate- or high-intensity infection category was defined as ≥ 1,000 epg. For A. lumbricoides, low- and moderate- or high-intensity infection categories were defined as 1–4,999 epg and ≥ 5,000 epg, respectively.
Cognitive tests. Cognitive tests were translated and adapted for use in The Philippines. They were then pilot tested among Filipino children from other S. japonicum- endemic villages near the study area. Reliability testing for each cognitive test included joint inter-rater and test-retest reliability with a six-week interval between tests. Assessment of the degree of internal consistency between respective tests that constitute the learning and memory domains of the Wide Range Assessment of Memory and Learning (WRAML) were assessed by Cronbach's alpha coefficient. For all cognitive tests, performance is categorized as high or low by the median of the distribution of test scores in each domain. A description of cognitive tests follows.
Wide Range Assessment of Memory and Learning. The WRAML was chosen so that we could address the effects of helminth infection on learning processes. This, as opposed to pure memory tests, may better reflect a child's ability to take advantage of limited educational opportunities. The WRAML was developed in the United States and was age standardized to a nationally representative sample of more than 2,300 children 5–17 years old.24 Specific subtests of the WRAML have been validated against school performance and have been shown to be significantly related to academic achievement in the subject areas they most closely mimic. For use in The Philippines, the WRAML was translated and then back-translated to check accuracy. We used two of the three cognitive domains, learning and verbal memory because the third domain, visual memory, proved too difficult to adapt. Two of the stories comprising the verbal memory subtest called story memory were adapted to better reflect, for example, things that might happen at a Filipino birthday party, rather than an American birthday party. The learning domain is defined by a combination of three WRAML subscales: verbal learning, sound symbol (auditory learning), and visual learning. The memory domain is also derived from three WRAML sub-scales: story memory, sentence memory, and number letter memory. For the memory and learning domains, age-standardized scores on the three subscales are added and a standardized score is created, which represents the final score used for the domain.
Verbal fluency. This test has been described as one likely to be an acceptable task across a wide range of cultures.25 A child is asked to name as many items in a given category as he or she can in 60 seconds after a practice category is given. Animals and things to eat were test categories. Verbal fluency is regarded as a good indicator of the operation of the central executive component of working memory.16,25
Philippines Non-Verbal Intelligence Test (PNIT). This test was adapted in The Philippines by American and Filipino psychologists. The test measures concept recognition and abstract thinking. It uses 100 cards each with 5 items depicted. Four of the five items capture a concept that the fifth does not. The child is asked to choose which item does not belong with the others. Reliability characteristics for this test were found to be excellent among a cohort of rural Filipino children (rho = 0.71–0.95 at six separate grade levels).26 Furthermore, the validity of this test was assessed against the grades that students had received in related courses. The validity coefficients obtained with the test were about the same as those obtained with cognitive tests given to American children.26
Mediating/confounding covariates. Socioeconomic status. Socioeconomic status was based on questionnaire data addressing parental and child educational status, occupation, ownership status of home/land, and assets. The questionnaire had good internal consistency with a Cronbach's alpha of 82.4% for all questions. Four domains of SES were determined a priori: educational status, social class position (occupation, land/home ownership, number of individuals living per room), material wealth, and a composite SES score using all questionnaire items. Scores for each of these four domains were calculated using principal components analysis to appropriately weight questionnaire items as described by Filmer and Pritchett.27 We assessed the construct validity of the summary SES score against nutritional status, which is known to be strongly related to SES and found a high correlation (rho = 0.309, P < 0.0001). The derived summary SES variable, a weighted combination of all items, was divided into low, medium and high by the tertiles of its distribution.
Body mass index (BMI) Z-score. Body mass index, which is weight in kilograms divided by height in meters squared, was calculated. The BMI Z-scores were also calculated using Epi-Info 2000 software (Centers for Disease Control and Prevention [CDC], Atlanta, GA). The Z-scores represent a given BMI's standard deviation from the age- and sex-adjusted CDC reference median from the year 2000.28
Statistical analyses. Separate logistic regression models were made for each cognitive domain to quantify the association between intensity of helminth infection and performance on cognitive tests. Uninfected children were the reference group for all analyses. Odds ratios (ORs) were estimated to quantify the odds of low performance on tests in respective domains for children with low- and moderate- or high-intensity infections compared with uninfected children; 95% confidence intervals (CIs) were also estimated to show range of values consistent with point estimates. Variables were considered confounders of the association between helminth infection and cognitive ability if they were independently associated with both S. japonicum infection and cognitive performance, and the OR derived by adjusting for them in bivariate models is up to 10% different from the crude OR. Potential confounders include sex, SES, nutritional status (BMI), and age. To explore the role of hemoglobin and nutritional status as mediators of S. japonicum-associated cognitive impairment, we built models with and without these variables and observed changes in point estimates and 95% CIs. Since both variables have been proposed as mediators of cognitive impairment in children, a lack of attenuation of the point estimates for the respective helminths, or the persistence of an association between helminth infection and cognitive performance after adjustment for these variables implies that differences in nutritional or hemoglobin status do not fully account for children's performance.
RESULTS
Results of reliability characteristics for cognitive tests are presented in Table 1. Inter-rater reliability (IRR) tests showed good consistency between raters of children's test scores (IRR ≥ 0.98 for all tests). Test-retest analyses showed good to excellent reliability (0.61–0.89). The degree of internal consistency among subscales of the WRAML memory domain and learning domain were good and fair, respectively.
Table 1.
Reliability of cognitive tests*
| Cognitive test | Inter-rater (joint reliability) | Test-retest | Cronbach alpha† |
|---|---|---|---|
| Memory domain of WRAML | 0.99 | 0.70 | 0.81 |
| Learning domain of WRAML | 0.99 | 0.79 | 0.54 |
| PNIT | 0.99 | 0.89 | NA |
| Verbal fluency | 0.98 | 0.61 | NA |
WRAML = Wide Range Assessment of Memory and Learning; PNIT = Philippine Non-Verbal Intelligence Test; NA = not applicable.
Cronbach's alpha for correlation among three subscales that constitute each domain.
Table 2 presents characteristics of the study cohort at baseline by intensity of S. japonicum infection. Overall, the prevalence of S. japonicum infection was 76.5%. Half (50.5%) of the children had low-intensity infections and slightly more than one-fourth (26%) had moderate- or high-intensity infections. The BMI Z-score did not differ by intensity of infection. Boys were over-represented among moderate- or high-intensity infected children (P < 0.0001). Children with moderate- or high-intensity S. japonicum infections, compared with their uninfected counterparts, were significantly more likely to have a low rather than a high SES (P = 0.012). Schistosoma japonicum–free children had, on average, significantly higher hemoglobin levels compared with both low- (P < 0.0001) and moderate- or high-intensity (P < 0.0001) S. japonicum-infected children. Schistosoma japonicum–infected children had significantly higher prevalences of A. lumbricoides and hookworm compared with their uninfected counterparts (P < 0.008).
Table 2.
Description of study population by Schistosoma japonicum infection intensity*
| ≥ Moderate intensity infection n = 83 (26.0%) |
||||||
|---|---|---|---|---|---|---|
| Variable | Whole cohort n = 319 (100%) Mean (SD) | S. japonicum infection free n = 75 (23.5%) Mean (SD) | Low intensity S. japonicum infection n = 161 (50.5%) Mean (SD) | Mean (SD) | P† | |
| Memory score | 65.4 (12.1) | 68.8 (13.8) | 65.5 (11.6) | 62 (10.81) | 0.001 | |
| Learning score | 87.8 (15.8) | 91.3 (15.5) | 88 (15.4) | 84 (16.1) | 0.003 | |
| PNIT score | 28.4 (7.7) | 27.8 (7.5) | 27.9 (7.8) | 29.5 (7.7) | 0.192 | |
| Verbal fluency score | 18.03 (5.01) | 17.4 (5.1) | 18.1 (4.6) | 18.0 (5.6) | 0.394 | |
| Age | 12.65 (3.00) | 12.9 (3.1) | 12.3 (2.9) | 13.0 (3.0) | 0.861 | |
| BMI Z-score | −1.1 (0.95) | −1.1 (0.8) | −1.1 (1.01) | −1.3 (0.95) | 0.214 | |
| Hemoglobin |
12.01 (1.71) |
12.7 (1.24) |
12.0 (1.4) |
11.2 (2.16) |
<0.001 |
|
| % |
% |
% |
P‡ |
% |
P§ |
|
| SES | ||||||
| % Low | 33.2 | 23.1 | 30.7 | 0.264 | 43.2 | 0.012 |
| % Medium | 35.2 | 33.9 | 31.4 | 0.727 | 28.4 | 0.487 |
| % High | 31.6 | 43.0 | 38.0 | 1.00 | 28.4 | 1.00 |
| Male | 57.0 | 45.3 | 52.8 | 0.286 | 75.9 | < 0.0001 |
| Parasite prevalence (%) | ||||||
| A. lumbricoides | 73.9 | 65.3 | 81.4 | 0.007 | 68.7 | 0.655 |
| T. trichiura | 92.2 | 88 | 92.6 | 0.253 | 95.2 | 0.101 |
| N. americanus | 45.8 | 30.7 | 49.1 | 0.008 | 51.8 | 0.007 |
PNIT = Philippine Non-Verbal Intelligence Test; BMI = body mass index; SES = socioeconomic status; A. = Ascaris; T. = Trichuris; N. = Necator.
P value based on analysis of variance test of differences in means of cognitive test scores, age, BMI-Z score, and hemoglobin, by intensity of S. japonicum.
P value based on chi-square test of differences in proportion of respective variables for children with low intensity S. japonicum infection relative to infection-free children.
P value based on chi-square test of differences in proportion of respective variables for children with moderate intensity S. japonicum infection or higher relative to infection-free children.
Table 3 presents bivariate associations between low performance in each of the cognitive tests and the following variables: hemoglobin, sex, nutritional status, age, SES, and four helminths from a logistic regression model. When unadjusted for any confounders, there were noteworthy patterns of association between performance in cognitive tests and sex, hemoglobin status, age, nutritional status, and helminth infection.
Table 3.
Poor performance in tests of cognitive ability among Filipino children and bivariate associations*
| Wide range assessment of memory and learning |
||||
|---|---|---|---|---|
| Covariates | Learning domain OR (95% CI) | Memory domain OR (95% CI) | Verbal fluency OR (95% CI) | PNIT OR (95% CI) |
| Sex (male) | 2.32 (1.49, 3.61) | 1.92 (1.24, 2.98) | 1.45 (0.94, 2.23) | 1.90 (1.17, 3.09) |
| Hemoglobin | 0.88 (0.77, 1.003) | 0.82 (0.71, 0.94) | 0.80 (0.70, 0.92) | 0.77 (0.66, 0.89) |
| Age (years) | 0.94 (0.87, 1.008) | 1.02 (0.95, 1.10) | 0.79 (0.73, 0.86) | 0.71 (0.64, 0.78) |
| BMI (kg/m2) | 0.87 (0.53, 1.40) | 0.90 (0.56, 1.45) | 0.39 (0.24, 0.64) | 0.17 (0.10, 0.29) |
| Socioeconomic status | ||||
| High | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 1.99 (1.13, 3.53) | 2.27 (1.28, 4.03) | 1.24 (0.71, 2.18) | 2.02 (1.12, 3.65) |
| Low | 3.67 (2.03, 6.64) | 3.44 (1.91, 6.20) | 2.11 (1.19, 3.73) | 3.90 (2.13, 7.13) |
| Schistosoma japonicum intensity | ||||
| Infection free | 1.00 | 1.00 | 1.00 | 1.00 |
| Low intensity | 1.93 (1.10, 3.37) | 1.25 (0.74, 2.09) | 0.86 (0.48, 1.56) | 0.94 (0.44, 2.05) |
| ≥ Moderate intensity | 3.29 (1.70, 6.33) | 2.02 (1.10, 3.72) | 1.35 (0.69, 2.63) | 1.14 (0.64, 2.81) |
| Necator americanus | ||||
| Infection free | 1.00 | 1.00 | 1.00 | 1.00 |
| Low intensity | 1.57 (0.99, 2.50) | 1.61 (1.02, 2.55) | 1.12 (0.71, 1.76) | 0.99 (0.60, 1.62) |
| ≥ Moderate intensity | 1.48 (0.63, 3.48) | 1.92 (0.81, 4.57) | 1.51 (0.59, 3.20) | 1.18 (0.48, 2.93) |
| Ascaris lumbricoides | ||||
| Infection free | 1.00 | 1.00 | 1.00 | 1.00 |
| Low intensity | 1.38 (0.74, 2.56) | 1.39 (0.76, 2.58) | 0.94 (0.50, 1.77) | 1.70 (0.79, 3.25) |
| ≥ Moderate intensity | 1.67 (0.99, 2.82) | 1.71 (1.02, 2.88) | 1.97 (1.17, 3.32) | 2.31 (1.27, 4.20) |
| Trichuris trichuria | ||||
| Infection free | 1.00 | 1.00 | 1.00 | 1.00 |
| Low intensity | 1.64 (0.70, 3.87) | 2.12 (0.88, 5.15) | 1.39 (0.76, 2.58) | 1.42 (0.29, 4.34) |
| ≥ Moderate intensity | 1.84 (0.80, 4.22) | 2.50 (1.06, 5.88) | 1.71 (1.02, 2.88) | 3.73 (1.26, 11.1) |
PNIT = Philippine Non-Verbal Intelligence Test; OR = odds ratio; CI = confidence interval; BMI = body mass index. All ORs and 95% CI quantify associations between shown variable and unadjusted odds of poor performance on various cognitive domain.
Mulivariable analyses. Table 4 shows the association between cognitive performance and helminth infections adjusted for known and potential confounders including age, SES, hemoglobin level, nutritional status and sex.
Table 4.
Multivariable adjusted associations between helminth infections and cognitive ability among Filipino children
| WRAML tests |
Non-WRAML tests |
|||
|---|---|---|---|---|
| Variable | Learning subscale OR (95% CI) | Memory subscale OR (95% CI) | Verbal fluency OR (95% CI) | PNIT OR (95% CI) |
| Sex (male) | 2.10 (1.21, 3.64) | 1.77 (1.01, 3.13) | 1.41 (0.81, 2.49) | 1.80 (0.96, 3.39) |
| Hemoglobin | 1.07 (0.90, 1.28) | 0.83 (0.68, 0.996) | 0.93 (0.77, 1.11) | 0.99 (0.76, 1.28) |
| Age (years) | 0.98 (0.86, 1.11) | 1.16 (1.05, 1.31) | 0.82 (0.75, 0.93) | 0.57 (0.46, 0.72) |
| BMI† (kg/m2) | 1.21 (0.60, 2.45) | 0.91 (0.68, 1.22) | 0.89 (0.67, 1.17) | 0.39 (0.19, 0.79) |
| SES | ||||
| High | 1.00 | 1.00 | 1.00 | 1.00 |
| Medium | 2.09 (1.10, 3.97) | 2.09 (1.10, 4.02) | 1.27 (0.65, 2.46) | 2.60 (1.25, 5.46) |
| Low | 3.79 (1.91, 7.50) | 3.57 (1.81, 7.16) | 1.09 (0.57, 2.10) | 3.90 (1.86, 8.40) |
| S. japonicum‡ | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| Low intensity | 1.89 (0.96, 3.76) | 0.98 (0.49, 1.92) | 0.56 (0.28, 1.14) | 1.27 (0.52, 3.15) |
| ≥ Moderate | 3.04 (1.37, 6.89) | 1.10 (0.49, 2.46) | 0.88 (0.39, 1.98) | 1.53 (0.70, 3.38) |
| A. lumbricoides | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| Low intensity | 1.37 (0.80, 3.55) | 1.37 (0.61, 3.13) | 0.94 (0.42, 2.11) | 1.40 (0.39, 5.00) |
| ≥ Moderate | 1.68 (0.68, 3.52) | 2.20 (1.04, 4.73) | 1.07 (0.51, 2.22) | 1.15 (0.37, 3.52) |
| N. americanus | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| Low intensity | 1.19 (0.66, 2.13) | 1.13 (0.65, 2.07) | 1.17 (0.66, 2.06) | 1.03 (0.46, 2.27) |
| ≥ Moderate | 0.64 (0.22, 1.89) | 0.56 (0.19, 1.69) | 1.54 (0.50, 4.72) | 0.88 (0.19, 4.09) |
| T. trichiura | ||||
| None | 1.00 | 1.00 | 1.00 | 1.00 |
| Low intensity | 0.59 (0.18, 1.97) | 1.15 (0.34, 3.84) | 4.32 (1.02, 31.4) | 0.51 (0.06, 4.24) |
| ≥ Moderate | 0.60 (0.18, 2.05) | 1.11 (0.32, 3.80) | 4.49 (1.04, 29.9) | 1.12 (0.14, 9.25) |
WRAML = Wide Range Assessment of Memory and Learning; PNIT = Philippine Non-Verbal Intelligence Test; OR = odds ratio; CI = confidence interval; BMI = body mass index; SES = socioeconomic status; S. = Schistosoma; A. = Ascaris; N. = Necator,; T. = Trichuris. All ORs and 95% CIs quantify the independent odds of poor performance on cognitive ability adjusted for the effect of confounders including sex, age SES, hemoglobin level, and co-existing parasites. Bold numbers indicate statistical significance.
BMI was dichotomized into high versus low categories by the mean BMI distribution. Low BMI is the reference category.
Odds of poor performance for specific infection intensity (or any intensity for S. japonicum) compared with uninfected.
Learning domain of WRAML. Children infected with S. japonicum at low intensity had odds of poor performance in the learning domain that was 1.9 times that of uninfected children (95% CI = 0.96–3.76), after adjusting for co-infection with the geohelminths and other confounders including SES, nutritional status, hemoglobin level, age, and sex. Similarly, children with moderate- or high-intensity S. japonicum infections had 3.04 times the odds of poor performance relative to uninfected children (95% CI = 1.04–6.9). The elevated odds of poor performance with schistosomiasis infection, although approaching statistical significance at all intensities, was significant only for moderate or high intensity relative to uninfected children. Boys and children in lower SES categories continued to have higher odds of low performance in the learning domain after adjustment for potential confounders.
Memory domain of WRAML. Schistosoma japonicum infection was not significantly related to poor performance in the memory domain of WRAML after adjusting for confounding variables. Moderate- or high-intensity infection with A. lumbricoides was positively and significantly associated with poor performance in cognitive tests of memory (OR = 2.2, 95% CI = 1.04–4.7). Other independent predictors of poor performance on tests of memory were hemoglobin, age, SES, and sex, with boys scoring lower than girls.
Verbal fluency. Infection with T. trichiura at any intensity relative to being uninfected was significantly associated with at least a four-fold higher odds of attaining a low score on tests of verbal fluency (Table 4). Children's performance on tests of verbal fluency did not differ significantly by S. japonicum infection, SES, nutritional status, hemoglobin level, or Ascaris or hookworm infection.
Philippine Non-Verbal Intelligence Test. Helminth infection, regardless of type and intensity of infection, was not associated with poor performance on the PNIT after confounder adjustment. However, nutritional status, age (the PNIT is not age standardized), and SES were strong inverse correlates of poor performance on the PNIT.
Mechanistic role of hemoglobin and nutritional status. To evaluate the mechanistic role of hemoglobin in mediating the relationship between helminth infections and cognitive impairment, multivariable models that controlled for age, SES, sex, nutritional status, and co-existing parasites with and without hemoglobin were evaluated. Changes in strength of association with inclusion/exclusion of hemoglobin in the logistic regression models are shown in Figure 1. The learning-schistosomiasis infection and the memory-Ascaris infection relationships were actually strengthened with hemoglobin in the respective regression models. When models were evaluated with and without BMI Z-score, the strength of relationship between respective helminth infection and cognitive function was relatively unchanged in all domains. For example, compared with estimate of associations in Table 4 that are adjusted for nutritional and hemoglobin status, removal of the BMI Z-score from the model leads to no changes in the memory domain (OR = 2.2, 95% CI = 1.03–4.9), a slight attenuation in the association between Trichuris and verbal fluency (OR = 4.3, 95% CI = 1.02–30), and a slight increase in association between S. japonicum and learning (OR = 3.1, 95% CI = 1.38–6.9).
Figure 1.
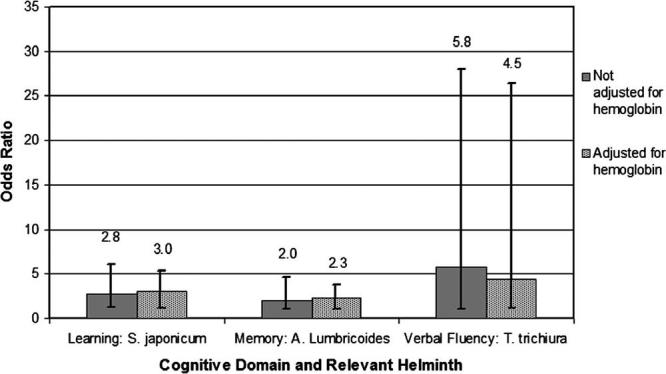
Mechanistic role of hemoglobin in cognitive impairment. Bars show odds ratios with 95% confidence intervals of the risk of performance below the median for those with moderate- or high-intensity infection versus uninfected for the designated helminth. S. = Schistosoma; A. = Ascaris; T. = Trichuris.
DISCUSSION
This study provides strong evidence of association between helminth infections and learning ability, memory, and verbal fluency in children after controlling for effects of important confounders including SES, nutritional status, age, sex, and presence of other intestinal parasites. To our knowledge, this is the first study to demonstrate a relationship between helminth infection and learning. We provide evidence that all intensities of S. japonicum infection, not just infections of moderate or high intensity, are associated with poorer performance on tests of learning. This is important because many children in endemic areas harbor only light infections, which may confer a learning disadvantage relative to uninfected children. Furthermore, in most areas of the developing world, educational opportunities are limited and effects on ability to learn in this age group may severely curtail attainment of educational potential. Our finding in the learning domain is congruent with what has been demonstrated in animal models for other kinds of helminth infections. In two such studies, infection with the helminths Strongyloides ratti and Toxocara canis were associated with poor performance on spatial learning tasks in mice.29,30
We are aware of only two human studies16,17 in Tanzania and China that reported associations between schistosomiasis infection and any domain of cognitive performance. Unlike our study, both groups found associations between tests of memory and schistosomiasis infection. Specifically, Jukes and others16 found that high-intensity infection with S. haematobium was associated with cognitive impairment in verbal and short-term memory domains among school age children in Tanzania. In a subset of younger children in China, Nokes and others17 found associations between treatment of S. japonicum and improvements in tests of memory (free recall, fluency, and picture search tests). We did not find this even when analyses were limited to younger children. The difference in domains of cognitive ability affected could be a reflection of the inherent challenges in quantifying constructs as complex as cognitive ability or the study design differences (cross-sectional versus randomized controlled trial).
Ascaris lumbricoides infection of moderate or high intensity was found to be associated with poor performance in tests relative to Ascaris-uninfected children in the memory domain. This finding is consistent with that of Nokes and others16 in China, Sakti and others31 in Indonesia, and Jukes and others17 in Tanzania. Watkins and others,32 in a randomized clinical trial among children in Guatemala, in which albendazole was administered at baseline and at three months for the treatment group, failed to find evidence of improvement in cognitive ability at six-months post-treatment of Ascaris infection. This finding is certainly possible in the context of their study, given that follow-up period was short and may not have been sufficient to detect any improvements that might have occurred. It is also possible that children require remedial assistance in addition to anti-helminthic therapy to observe improvements in cognitive performance.
This study provides additional support for a relationship between T. trichiura infection and poor performance in tests of verbal fluency. Specifically, we found that children infected with Trichuris had almost 4.5 times greater odds of performing poorly in tests of verbal fluency regardless of infection intensity. This finding is congruent with those of other investigators in Jamaican children,33-37 in which improvements in tests of verbal fluency were noted among children treated for moderate or high intensities of Trichuris infection.
Several investigators have demonstrated that helminth infections are associated with poor nutritional status and lower hemoglobin levels or anemia.38-44 Positive associations between higher cognitive performance and better nutritional and hemoglobin status have also been reported.18,20,45-49 Our finding that hemoglobin and nutritional status were inversely related to cognitive performance in the memory and PNIT domains, respectively, is consistent with these previous research findings. Furthermore, this study underscores the importance of rigorous control for confounding variables in the examination of the relationship between helminth infection and cognitive performance. We found SES to be strongest and most consistent correlate of cognitive impairment. This finding is not surprising since higher SES may reflect among other things, access to superior educational and health care opportunities that may be beneficial to children's performance in cognitive tests. Failure to robustly control for SES could result in spurious associations or over-estimation of the true relationship between helminth infection and cognitive performance. In addition, sex was a confounder of these relationships since boys were more intensely infected and performed worse on tests of cognition, especially the WRAML. We explored the potential for effect modification of the effect of helminth on cognitive ability by sex to see if there is a stronger relationship between cognitive impairment and helminth infection among boys. We failed to find evidence for this from our analyses and therefore conclude that helminth infection affects cognitive performance similarly among boys and girls.
Because the relationship between hemoglobin levels and cognitive performance has been well described in the literature and investigators have postulated that cognitive impairment in the context of helminth infection may be mediated through anemia, we built models with and without hemoglobin to see if the effects of parasitic infection on cognitive ability was mediated through hemoglobin. With inclusion of hemoglobin, we expected changes in point estimates to include the null value in the 95% CI if most of the effects on cognitive ability is mediated through this covariate (Figure 1). We found an increase, rather then a decrease, in the strength of association between cognitive ability (learning and memory) and infection with S. japonicum and A. lumbricoides upon adjustment for hemoglobin status. The observation of an 8–10% increase in the strength of association suggests that hemoglobin, although independently associated with cognitive ability, is unlikely to be the dominant mediator of cognitive impairment in the context of S. japonicum and A. lubmricoides. In addition, inclusion of nutritional status in multivariable models did not change observed associations, suggesting under-nutrition is not the primary mediator of the observed relationships. Other potential mediating factors may include pro-inflammatory cytokines made in response to infection,50,51 which are known to have a detrimental effect on cognitive function.52,53 In particular, serum tumor necrosis factor-α levels are higher in individuals with schistosomiasis infection,54,55 and this cytokine has been related to decreased performance on cognitive tests in human subjects.53 In addition, abdominal discomfort related to A. lumbricoides, S. japonicum, and T. trichiura infection could affect cognitive performance if significant enough to distract children from cognitive tasks. For the Trichuris-verbal fluency relationship on the other hand, we observed 22.4% diminution in the strength of association upon adjustment for hemoglobin. This observation suggests that hemoglobin may be playing a more significant mechanistic role in the T. trichiura-fluency relationship.
The findings presented must be interpreted in light of limitations inherent in cross-sectional study design. First, the cross-sectional design does not allow causal inferences to be made. Although it is possible that cognitive impairment predisposes children to helminth infections, we believe reverse causality is unlikely to explain these findings since SES, not cognitive ability, is more likely to increase children's helminth exposure. We have adjusted for SES, which is directly correlated with cognitive function, and is one of the most important factors related to helminth exposure. Analysis of longitudinal data from this population, to be presented elsewhere, will increase our causal inference in addition to decoupling the temporal relationship between exposure to helminth infection and cognitive performance.
In addition, although we have adjusted for SES and other important confounders, it is possible that there are residual confounding or unmeasured confounding variables that could explain these relationships. Furthermore, it is possible that after the adaptation process of the WRAML, it may not capture constructs such as learning in the same way as when it used in populations in the United States, which may be more similar to the population in which the test was validated. We believe this is unlikely since only minor adaptations were made, and this was done only to replace unfamiliar customs or items with those of a similar level of difficulty. Finally, simultaneous exposure to multiple parasite species is common in our study population, as is the case in most helminth-endemic regions. It is possible that biologic interactions occur among co-infecting species, potentially modifying the association between any helminth species and cognitive performance. It remains an empirical challenge to quantify the effect of poly-parasite helminth exposures on helminth-associated morbidities without simultaneously sacrificing statistical and inferential efficiency given the co-linear distribution of helminths under investigation and the potential for interaction.
We have provided evidence that helminth infection is associated with cognitive impairment in school age children. We found impairments in learning, memory, and verbal fluency for different helminths. It is not surprising that different parasites were associated with deficits in different domains, since these infections cause vastly different host responses based on their location and parasite-specific immunologic and hematologic effects. Given the resource-poor nature of helminth-endemic regions, prioritization of public health interventions that are most cost effective is crucial. Defining the global burden of helminth infections entails assessing more subtle morbidities.56 These morbidities, particularly when clinically apparent at low intensities of infection that are highly prevalent, likely contribute significantly to disability-adjusted life years.7
Acknowledgments
We thank our field staff for their diligence and energy: Blanca Jarilla, Mario Jiz, Archie Pablo, Raquel Pacheco, Patrick Sebial, Mary Paz Urbina, and Jemaima Yu. We also thank the study participants from Macanip, Buri, and Pitogo in Leyte, The Philippines.
Footnotes
Financial support: This work supported by National Institutes of Health grants RO1 AI48123 and K23 AI52125.
REFERENCES
- 1.World Health Organization Prevention and control of intestinal parasitic infections. World Health Organ Tech Rep Ser. 1997;749:1–86. [PubMed] [Google Scholar]
- 2.Stephenson LS, Holland CV, Cooper ES. The public health significance of Trichuris trichiura. Parasitology. 2000;121(Suppl):S73–S95. doi: 10.1017/s0031182000006867. [DOI] [PubMed] [Google Scholar]
- 3.Hotez PJ, Zhan B, Bethony JM, Loukas A, Williamson A, Goud GN, Hawdon JM, Dobardzic A, Dobardzic R, Ghosh K, Bottazzi ME, Mendez S, Zook B, Wang Y, Liu S, Essiet-Gibson I, Chung-Debose S, Xiao S, Knox D, Meagher M, Inan M, Correa-Oliveira R, Vilk P, Shepherd HR, Brandt W, Russell PK. Progress in the development of a recombinant vaccine for human hookworm disease: The Human Hookworm Vaccine Initiative. Int J Parasitol. 2003;33:1245–1258. doi: 10.1016/s0020-7519(03)00158-9. [DOI] [PubMed] [Google Scholar]
- 4.Smith H, Dekaminsky R, Niwas S, Soto R, Jolly P. Prevalence and intensity of infections of Ascaris lumbricoides and Trichuris trichiura and associated socio-demographic variables in four rural Honduran communities. Mem Inst Oswaldo Cruz. 2001;96:303–314. doi: 10.1590/s0074-02762001000300004. [DOI] [PubMed] [Google Scholar]
- 5.Leonardo LR, Acosta LP, Olveda RM, Aligui GD. Difficulties and strategies in the control of schistosomiasis in the Philipines. Acta Trop. 2002;82:295–299. doi: 10.1016/s0001-706x(02)00022-0. [DOI] [PubMed] [Google Scholar]
- 6.Cabrera BD. Reinfection and infection rates of ascariasis in relation to seasonal variation in the Philippines. Southeast Asian J Trop Med Public Health. 1984;15:394–401. [PubMed] [Google Scholar]
- 7.World Bank . World Development Report: Investing in Health. Oxford University Press; Oxford, United Kingdom: 1993. 1993. [Google Scholar]
- 8.Drake LJ, Bundy DA. Multiple helminth infections in children: impact and control. Parasitology. 2001;122(Suppl):S73–S81. doi: 10.1017/s0031182000017662. [DOI] [PubMed] [Google Scholar]
- 9.Stephenson LS, Latham MC, Ottesen EA. Global malnutrition. Parasitology. 2000;121(Suppl):S5–22. doi: 10.1017/s0031182000006478. [DOI] [PubMed] [Google Scholar]
- 10.Stephenson LS, Latham MC, Ottesen EA. Malnutrition and parasitic helminth infections. Parasitology. 2000;121(Suppl):S23–S38. doi: 10.1017/s0031182000006491. [DOI] [PubMed] [Google Scholar]
- 11.Stoltzfus RJ, Albonico M, Chwaya HM, Savioli L, Tielsch J, Schulze K, Yip R. Hemoquant determination of hookworm-related blood loss and its role in iron deficiency in African children. Am J Trop Med Hyg. 1996;55:399–404. doi: 10.4269/ajtmh.1996.55.399. [DOI] [PubMed] [Google Scholar]
- 12.Stoltzfus RJ, Chwaya HM, Tielsch JM, Schulze KJ, Albonico M, Savioli L. Epidemiology of iron deficiency anemia in Zanzibari schoolchildren: the importance of hookworms. Am J Clin Nutr. 1997;65:153–159. doi: 10.1093/ajcn/65.1.153. [DOI] [PubMed] [Google Scholar]
- 13.Dickson R, Awasthi S, Williamson P, Demellweek C, Garner P. Effects of treatment for intestinal helminth infection on growth and cognitive performance in children: systematic review of randomised trials. BMJ. 2000;320:1697–1701. doi: 10.1136/bmj.320.7251.1697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hadidjaja P, Bonang E, Suyardi MA, Abidin SA, Ismid IS, Margono SS. The effect of intervention methods on nutritional status and cognitive function of primary school children infected with Ascaris lumbricoides. Am J Trop Med Hyg. 1998;59:791–795. doi: 10.4269/ajtmh.1998.59.791. [DOI] [PubMed] [Google Scholar]
- 15.Thein-Hlaing, Thane-Toe, Than-Saw, Myat-Lay-Kyin, Myint-Lwin A controlled chemotherapeutic intervention trial on the relationship between Ascaris lumbricoides infection and malnutrition in children. Trans R Soc Trop Med Hyg. 1991;85:523–528. doi: 10.1016/0035-9203(91)90242-q. [DOI] [PubMed] [Google Scholar]
- 16.Nokes C, McGarvey ST, Shiue L, Wu G, Wu H, Bundy DA, Olds GR. Evidence for an improvement in cognitive function following treatment of Schistosoma japonicum infection in Chinese primary schoolchildren. Am J Trop Med Hyg. 1999;60:556–565. doi: 10.4269/ajtmh.1999.60.556. [DOI] [PubMed] [Google Scholar]
- 17.Jukes MC, Nokes CA, Alcock KJ, Lambo JK, Kihamia C, Ngorosho N, Mbise A, Lorri W, Yona E, Mwanri L, Baddeley AD, Hall A, Bundy DA, Partnership for Child Development Heavy schistosomiasis associated with poor short-term memory and slower reaction times in Tanzanian schoolchildren. Trop Med Int Health. 2002;7:104–117. doi: 10.1046/j.1365-3156.2002.00843.x. [DOI] [PubMed] [Google Scholar]
- 18.Boivin MJ, Giordani B. Improvements in cognitive performance for schoolchildren in Zaire, Africa, following an iron supplement and treatment for intestinal parasites. J Pediatr Psychol. 1993;18:249–264. doi: 10.1093/jpepsy/18.2.249. [DOI] [PubMed] [Google Scholar]
- 19.Boivin MJ, Giordani B, Ndanga K, Maky MM, Manzeki KM, Ngunu N. Economic advantage and the cognitive ability of rural children in Zaire. J Psychol. 1996;130:95–107. doi: 10.1080/00223980.1996.9914992. [DOI] [PubMed] [Google Scholar]
- 20.Boivin MJ, Giordani B, Ndanga K, Maky MM, Manzeki KM, Ngunu N, Muamba K. Effects of treatment for intestinal parasites and malaria on the cognitive abilities of schoolchildren in Zaire, Africa. Health Psychol. 1993;12:220–226. doi: 10.1037//0278-6133.12.3.220. [DOI] [PubMed] [Google Scholar]
- 21.Nokes C. A healthy body and a healthy mind?: the relationship between ill-health and cognitive function in school-age children. J Biosoc Sci. 1996;28:453–462. doi: 10.1017/s0021932000022525. [DOI] [PubMed] [Google Scholar]
- 22.Olveda RM, Daniel BL, Ramirez BD, Aligui GD, Acosta LP, Fevidal P, Tiu E, de Veyra F, Peters PA, Romulo R, Domingo E, Wiest PM, Olds GR. Schistosomiasis japonica in the Philippines: the long-term impact of population-based chemotherapy on infection, transmission, and morbidity. J Infect Dis. 1996;174:163–172. doi: 10.1093/infdis/174.1.163. [DOI] [PubMed] [Google Scholar]
- 23.Olveda RM, Tiu E, Fevidal P, Jr, de Veyra F, Jr, Icatlo FC, Jr, Domingo EO. Relationship of prevalence and intensity of infection to morbidity in schistosomiasis japonica: a study of three communities in Leyte, Philippines. Am J Trop Med Hyg. 1983;32:1312–1321. doi: 10.4269/ajtmh.1983.32.1312. [DOI] [PubMed] [Google Scholar]
- 24.Adams W, Sheslow D. Wide Range Assessment of Memory and Learning. Jastak Associates; Wilmington, DE: 1990. [Google Scholar]
- 25.Baddeley A, Gardner JM, Grantham-McGregor S. Cross-cultural cognition: developing tests for developing countries. Appl Cognitive Psychol. 1995;9:S173–S195. [Google Scholar]
- 26.Guthrie GM, Tayag AH, Jimenez-Jacobs P. The Philippine nonverbal intelligence test. J Soc Psychol. 1977;102:3–11. [Google Scholar]
- 27.Filmer D, Pritchett LH. Estimating wealth effects without expenditure data–or tears: an application to educational enrollments in states of India. Demography. 2001;38:115–132. doi: 10.1353/dem.2001.0003. [DOI] [PubMed] [Google Scholar]
- 28.Ogden CL, Kuczmarski RJ, Flegal KM, Mei Z, Guo S, Wei R, Grummer-Strawn LM, Curtin LR, Roche AF, Johnson CL. Centers for Disease Control and Prevention 2000 growth charts for the United States: improvements to the 1977 National Center for Health Statistics version. Pediatrics. 2002;109:45–60. doi: 10.1542/peds.109.1.45. [DOI] [PubMed] [Google Scholar]
- 29.Braithwaite VA, Salkeld DJ, McAdam HM, Hockings CG, Ludlow AM, Read AF. Spatial and discrimination learning in rodents infected with the nematode Strongyloides ratti. Parasitology. 1998;117:145–154. doi: 10.1017/s003118209800290x. [DOI] [PubMed] [Google Scholar]
- 30.Cox DM, Holland CV. Relationship between three intensity levels of Toxocara canis larvae in the brain and effects on exploration, anxiety, learning and memory in the murine host. J Helminthol. 2001;75:33–41. doi: 10.1079/joh200028. [DOI] [PubMed] [Google Scholar]
- 31.Sakti H, Nokes C, Hertanto WS, Hendratno S, Hall A, Bundy DA, Satoto Evidence for an association between hookworm infection and cognitive function in Indonesian school children. Trop Med Int Health. 1999;4:322–334. doi: 10.1046/j.1365-3156.1999.00410.x. [DOI] [PubMed] [Google Scholar]
- 32.Watkins WE, Cruz JR, Pollitt E. The effects of deworming on indicators of school performance in Guatemala. Trans R Soc Trop Med Hyg. 1996;90:156–161. doi: 10.1016/s0035-9203(96)90121-2. [DOI] [PubMed] [Google Scholar]
- 33.Simeon DT, Grantham-McGregor SM, Callender JE, Wong MS. Treatment of Trichuris trichiura infections improves growth, spelling scores and school attendance in some children. J Nutr. 1995;125:1875–1883. doi: 10.1093/jn/125.7.1875. [DOI] [PubMed] [Google Scholar]
- 34.Sternberg RJ, Powell C, McGrane P, Grantham-McGregor S. Effects of a parasitic infection on cognitive functioning. J Exp Psychol: Applied. 1997;3:67–76. [Google Scholar]
- 35.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Robinson BA, Bundy DA. Moderate to heavy infections of Trichuris trichiura affect cognitive function in Jamaican school children. Parasitology. 1992;104:539–547. doi: 10.1017/s0031182000063800. [DOI] [PubMed] [Google Scholar]
- 36.Nokes C, Grantham-McGregor SM, Sawyer AW, Cooper ES, Bundy DA. Parasitic helminth infection and cognitive function in school children. Proc R Soc Lond B Biol Sci. 1992;247:77–81. doi: 10.1098/rspb.1992.0011. [DOI] [PubMed] [Google Scholar]
- 37.Nokes C, Cooper ES, Robinson BA, Bundy DA. Geohelminth infection and academic assessment in Jamaican children. Trans R Soc Trop Med Hyg. 1991;85:272–273. doi: 10.1016/0035-9203(91)90052-z. [DOI] [PubMed] [Google Scholar]
- 38.McGarvey ST, Wu G, Zhang S, Wang Y, Peters P, Olds GR, Wiest PM. Child growth, nutritional status, and schistosomiasis japonica in Jiangxi, People's Republic of China. Am J Trop Med Hyg. 1993;48:547–553. doi: 10.4269/ajtmh.1993.48.547. [DOI] [PubMed] [Google Scholar]
- 39.Hall A. Nutritional aspects of parasitic infection. Prog Food Nutr Sci. 1985;9:227–256. [PubMed] [Google Scholar]
- 40.Blackburn HD, Rocha JL, Figueiredo EP, Berne ME, Vieira LS, Cavalcante AR, Rosa JS. Interaction of parasitism and nutrition and their effects on production and clinical parameters in goats. Vet Parasitol. 1991;40:99–112. doi: 10.1016/0304-4017(91)90086-b. [DOI] [PubMed] [Google Scholar]
- 41.Hlaing T. Ascariasis and childhood malnutrition. Parasitology. 1993;107(Suppl):S125–S136. doi: 10.1017/s0031182000075557. [DOI] [PubMed] [Google Scholar]
- 42.Lunn PG, Northrop-Clewes CA. The impact of gastrointestinal parasites on protein-energy malnutrition in man. Proc Nutr Soc. 1993;52:101–111. doi: 10.1079/pns19930042. [DOI] [PubMed] [Google Scholar]
- 43.Hagel I, Lynch NR, Di Prisco MC, Perez M, Sanchez JE, Pereyra BN, Soto de Sanabria I. Helminthic infection and anthropometric indicators in children from a tropical slum: Ascaris reinfection after anthelmintic treatment. J Trop Pediatr. 1999;45:215–220. doi: 10.1093/tropej/45.4.215. [DOI] [PubMed] [Google Scholar]
- 44.Zulkifli A, Anuar AK, Atiya AS, Yano A. The prevalence of malnutrition and geo-helminth infections among primary schoolchildren in rural Kelantan. Southeast Asian J Trop Med Public Health. 2000;31:339–345. [PubMed] [Google Scholar]
- 45.van Stuijvenberg ME, Kvalsvig JD, Faber M, Kruger M, Kenoyer DG, Benade AJ. Effect of iron-, iodine-, and beta-carotene-fortified biscuits on the micronutrient status of primary school children: a randomized controlled trial. Am J Clin Nutr. 1999;69:497–503. doi: 10.1093/ajcn/69.3.497. [DOI] [PubMed] [Google Scholar]
- 46.Scrimshaw NS. Iron deficiency. Sci Am. 1991;265:46–52. doi: 10.1038/scientificamerican1091-46. [DOI] [PubMed] [Google Scholar]
- 47.Brown WS, Marsh JT, Wolcott D, Takushi R, Carr CR, Higa J, Nissenson AR. Cognitive function, mood and P3 latency: effects of the amelioration of anemia in dialysis patients. Neuropsychologia. 1991;29:35–45. doi: 10.1016/0028-3932(91)90092-m. [DOI] [PubMed] [Google Scholar]
- 48.Deinard AS, List A, Lindgren B, Hunt JV, Chang PN. Cognitive deficits in iron-deficient and iron-deficient anemic children. J Pediatr. 1986;108:681–689. doi: 10.1016/s0022-3476(86)81041-1. [DOI] [PubMed] [Google Scholar]
- 49.Stephenson LS. Helminth parasites, a major factor in malnutrition. World Health Forum. 1994;15:169–172. [PubMed] [Google Scholar]
- 50.Zwingenberger K, Richter J, Taupitz S, Vergetti Siqueira JG, Correia Dacal AR. Altered generation of interleukin 1 in chronic human schistosomiasis mansoni. Scand J Immunol. 1990;31:729–736. doi: 10.1111/j.1365-3083.1990.tb02824.x. [DOI] [PubMed] [Google Scholar]
- 51.Zwingenberger K, Irschick E, Vergetti Siqueira JG, Correia Dacal AR, Feldmeier H. Tumour necrosis factor in hepatosplenic schistosomiasis. Scand J Immunol. 1990;31:205–211. doi: 10.1111/j.1365-3083.1990.tb02761.x. [DOI] [PubMed] [Google Scholar]
- 52.Smith A, Tyrrell D, Coyle K, Higgins P. Effects of interferon alpha on performance in man: a preliminary report. Psychopharmacology (Berl) 1988;96:414–416. doi: 10.1007/BF00216072. [DOI] [PubMed] [Google Scholar]
- 53.Reichenberg A, Yirmiya R, Schuld A, Kraus T, Haack M, Morag A, Pollmacher T. Cytokine-associated emotional and cognitive disturbances in humans. Arch Gen Psychiatry. 2001;58:445–452. doi: 10.1001/archpsyc.58.5.445. [DOI] [PubMed] [Google Scholar]
- 54.Abdel Azim A, Sedky HA, el-Tahawy MA, Fikry AA, Mostafa H. Serum levels of tumor necrosis factor in different stages of schistosomal infection. J Egypt Soc Parasitol. 1995;25:279–287. [PubMed] [Google Scholar]
- 55.Mwatha JK, Kimani G, Kamau T, Mbugua GG, Ouma JH, Mumo J, Fulford AJ, Jones FM, Butterworth AE, Roberts MB, Dunne DW. High levels of TNF, soluble TNF receptors, soluble ICAM-1, and IFN-gamma, but low levels of IL-5, are associated with hepatosplenic disease in human schistosomiasis mansoni. J Immunol. 1998;160:1992–1999. [PubMed] [Google Scholar]
- 56.McGarvey ST. Schistosomiasis: impact on childhood and adolescent growth, malnutrition and morbidity. Semin Pediatr Infect Dis. 2000;11:269–274. [Google Scholar]


