Abstract
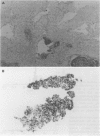
The gland isolation method was applied to various gastric lesions to measure DNA content by cytophotometry and flow cytometry for the first time. By incubating and agitating fresh specimens from surgically resected stomachs in calcium-magnesium free Hanks's balanced salt solution (CMFH) containing EDTA, many neoplastic glandular epithelial cells were successfully isolated from the stroma, and their characteristic three dimensional features were seen morphologically. The DNA content of pure nuclear suspensions of isolated glands was obtained by cytophotometry and flow cytometry staining with 4',6-diamino-2-phenylindole dihydrochloride (DAPI) and propidium iodide, respectively. Compared with histological grading, the frequency of the DNA aneuploidy of cancer with moderate or poor differentiation by cytophotometry (75%) was significantly higher than that of well differentiated cancer (25%), but the histological typing of gastric cancer DNA frequency were not correlated. This method allowed us to detect small aneuploid peaks by flow cytometry, which were previously masked by contaminating interstitial cells. The frequency of DNA aneuploidy detected by flow cytometry (87.5%) was higher than detected by cytophotometry (58.3%). The results of these studies shows the feasibility of this technique for analysing the DNA content of various lesions of the stomach.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arai T., Kino I. Morphometrical and cell kinetic studies of normal human colorectal mucosa. Comparison between the proximal and the distal large intestine. Acta Pathol Jpn. 1989 Nov;39(11):725–730. doi: 10.1111/j.1440-1827.1989.tb02421.x. [DOI] [PubMed] [Google Scholar]
- Baretton G., Gille J., Oevermann E., Löhrs U. Flow-cytometric analysis of the DNA-content in paraffin-embedded tissue from colorectal carcinomas and its prognostic significance. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(2):123–131. doi: 10.1007/BF02899537. [DOI] [PubMed] [Google Scholar]
- Bjerknes M., Cheng H. Methods for the isolation of intact epithelium from the mouse intestine. Anat Rec. 1981 Apr;199(4):565–574. doi: 10.1002/ar.1091990412. [DOI] [PubMed] [Google Scholar]
- Cheng H., Bjerknes M., Amar J. Methods for the determination of epithelial cell kinetic parameters of human colonic epithelium isolated from surgical and biopsy specimens. Gastroenterology. 1984 Jan;86(1):78–85. [PubMed] [Google Scholar]
- Cheng H., Bjerknes M. Whole population cell kinetics and postnatal development of the mouse intestinal epithelium. Anat Rec. 1985 Apr;211(4):420–426. doi: 10.1002/ar.1092110408. [DOI] [PubMed] [Google Scholar]
- Dupont C., Laburthe M., Broyart J. P., Bataille D., Rosselin G. Cyclic AMP production in isolated colonic epithelial crypts: a highly sensitive model for the evaluation of vasoactive intestinal peptide action in human intestine. Eur J Clin Invest. 1980 Feb;10(1):67–76. doi: 10.1111/j.1365-2362.1980.tb00012.x. [DOI] [PubMed] [Google Scholar]
- Fausel R. E., Burleigh W., Kaminsky D. B. DNA quantification in colorectal carcinoma using flow and image analysis cytometry. Anal Quant Cytol Histol. 1990 Feb;12(1):21–27. [PubMed] [Google Scholar]
- Freeman H. J., San R. H. Use of unscheduled DNA synthesis in freshly isolated human intestinal mucosal cells for carcinogen detection. Cancer Res. 1980 Sep;40(9):3155–3157. [PubMed] [Google Scholar]
- Hamada S., Itoh R., Fujita S. DNA distribution pattern of the so-called severe dysplasias and small carcinomas of the colon and rectum and its possible significance in the tumor progression. Cancer. 1988 Apr 15;61(8):1555–1562. doi: 10.1002/1097-0142(19880415)61:8<1555::aid-cncr2820610812>3.0.co;2-w. [DOI] [PubMed] [Google Scholar]
- Hamada S., Namura K., Itoh R., Fujita S. Characteristics of colorectal epithelia and adenomas as revealed by DNA cytofluorometry. Jpn J Cancer Res. 1987 Aug;78(8):826–832. [PubMed] [Google Scholar]
- Hamada S., Namura K., Itoh R., Fujita S. Characteristics of colorectal epithelia and adenomas as revealed by DNA cytofluorometry. Jpn J Cancer Res. 1987 Aug;78(8):826–832. [PubMed] [Google Scholar]
- Hedley D. W., Friedlander M. L., Taylor I. W., Rugg C. A., Musgrove E. A. Method for analysis of cellular DNA content of paraffin-embedded pathological material using flow cytometry. J Histochem Cytochem. 1983 Nov;31(11):1333–1335. doi: 10.1177/31.11.6619538. [DOI] [PubMed] [Google Scholar]
- Kaern J., Wetteland J., Tropé C. G., Farrants G. W., Juhng S. W., Pettersen E. O., Reith A., Danielsen H. E. Comparison between flow cytometry and image cytometry in ploidy distribution assessments in gynecologic cancer. Cytometry. 1992;13(3):314–321. doi: 10.1002/cyto.990130314. [DOI] [PubMed] [Google Scholar]
- Kallioniemi O. P. Comparison of fresh and paraffin-embedded tissue as starting material for DNA flow cytometry and evaluation of intratumor heterogeneity. Cytometry. 1988 Mar;9(2):164–169. doi: 10.1002/cyto.990090211. [DOI] [PubMed] [Google Scholar]
- Kemler B. J., Alpert E. Inflammatory bowel disease: study of cell mediated cytotoxicity for isolated human colonic epithelial cells. Gut. 1980 May;21(5):353–359. doi: 10.1136/gut.21.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macartney J. C., Camplejohn R. S. DNA flow cytometry of histological material from dysplastic lesions of human gastric mucosa. J Pathol. 1986 Oct;150(2):113–118. doi: 10.1002/path.1711500205. [DOI] [PubMed] [Google Scholar]
- Merkel D. E., McGuire W. L. Ploidy, proliferative activity and prognosis. DNA flow cytometry of solid tumors. Cancer. 1990 Mar 1;65(5):1194–1205. doi: 10.1002/1097-0142(19900301)65:5<1194::aid-cncr2820650528>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- Nakamura S., Kino I., Baba S. Nuclear DNA content of isolated crypts of background colonic mucosa from patients with familial adenomatous polyposis and sporadic colorectal cancer. Gut. 1993 Sep;34(9):1240–1244. doi: 10.1136/gut.34.9.1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roediger W. E., Truelove S. C. Method of preparing isolated colonic epithelial cells (colonocytes) for metabolic studies. Gut. 1979 Jun;20(6):484–488. doi: 10.1136/gut.20.6.484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vindeløv L. L., Christensen I. J. A review of techniques and results obtained in one laboratory by an integrated system of methods designed for routine clinical flow cytometric DNA analysis. Cytometry. 1990;11(7):753–770. doi: 10.1002/cyto.990110702. [DOI] [PubMed] [Google Scholar]
- Weiss H., Gütz H. J., Schröter J., Wildner G. P. DNA distribution pattern in chronic gastritis. I. DNA ploidy and cell cycle distribution. Scand J Gastroenterol. 1989 Aug;24(6):643–648. doi: 10.3109/00365528909093103. [DOI] [PubMed] [Google Scholar]
- Whitehead R. H., Brown A., Bhathal P. S. A method for the isolation and culture of human colonic crypts in collagen gels. In Vitro Cell Dev Biol. 1987 Jun;23(6):436–442. doi: 10.1007/BF02623860. [DOI] [PubMed] [Google Scholar]