Abstract
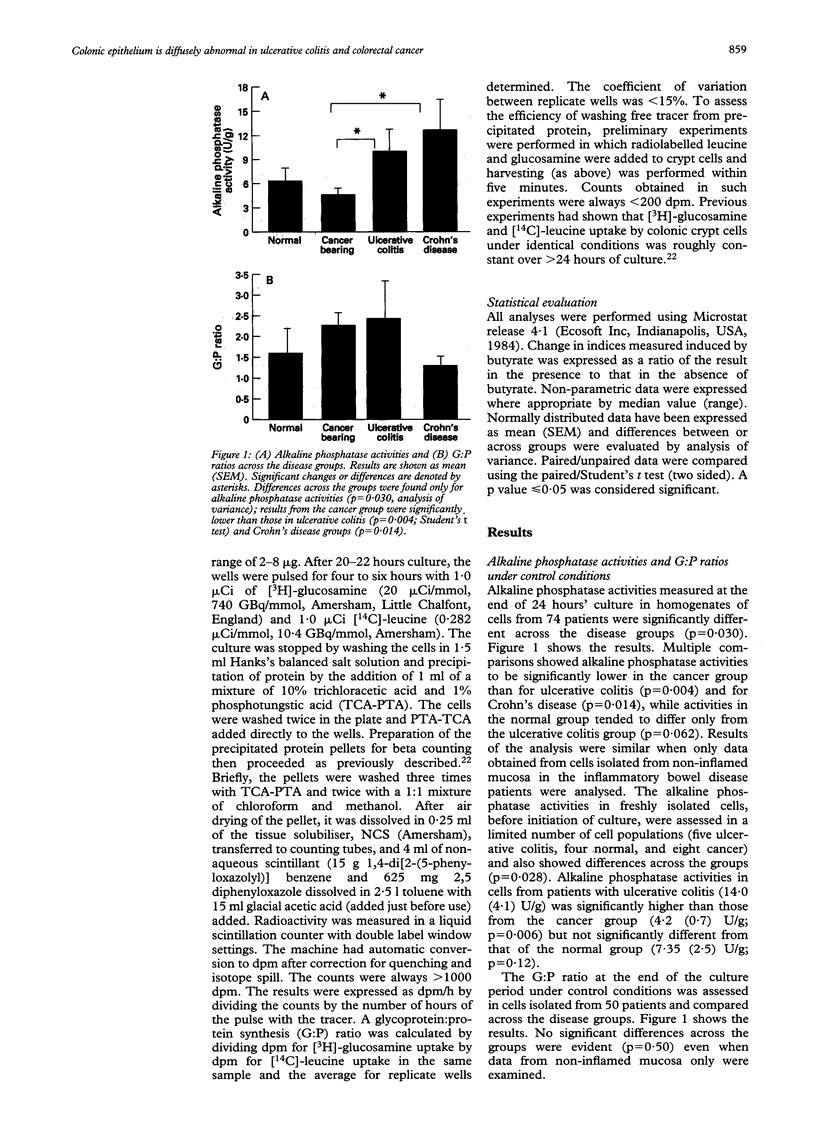
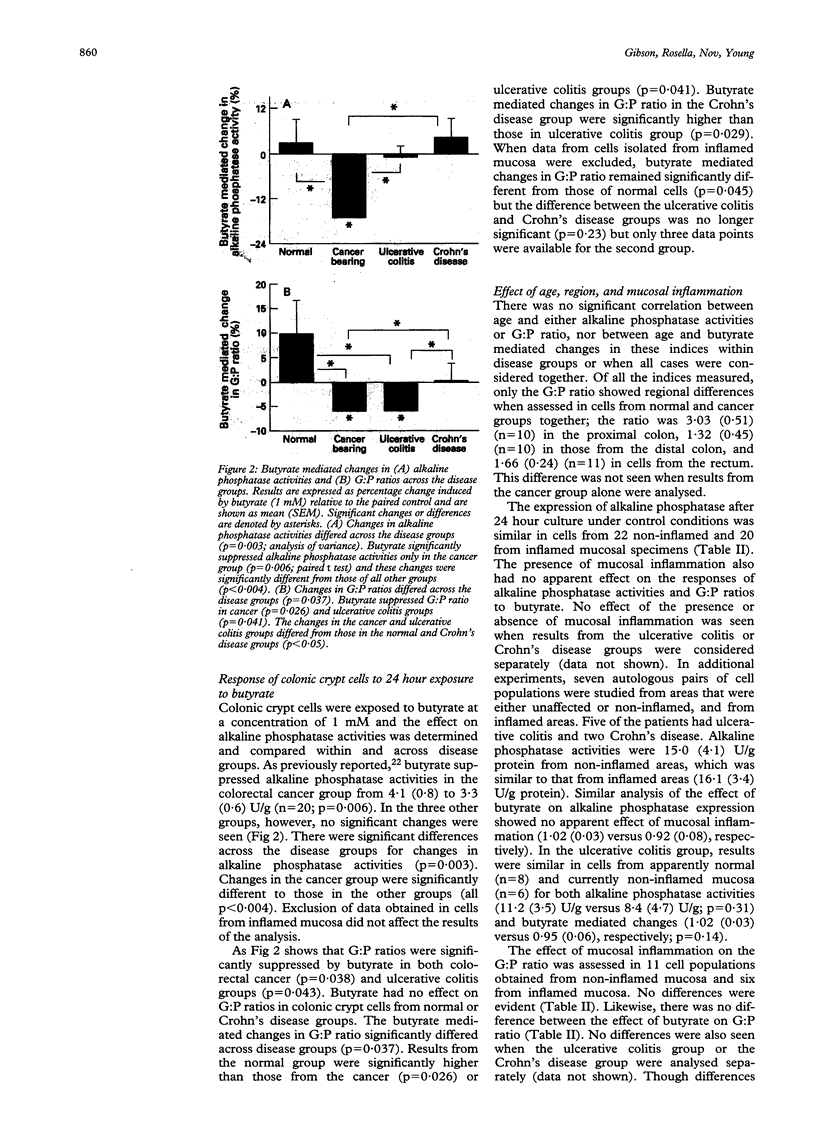
The hypothesis that the colonic epithelium is diffusely abnormal in ulcerative colitis was examined by comparing disease related responses in expression of markers of differentiation by colonic crypt cells to culture with and without butyrate. Cells were isolated from patients with normal colon (15), cancer (24), ulcerative colitis (19), or Crohn's disease (16). Alkaline phosphatase activities were measured in cell homogenates and the rate of glycoprotein synthesis assessed at the end of 24 hours of culture and expressed relative to the rate of protein synthesis as the G:P ratio. Alkaline phosphatase activities, but not G:P ratios, differed across the groups before and after 24 hour culture (p < 0.05), activities being lowest in the cancer group and highest in inflammatory bowel disease groups. Butyrate (1 mM) suppressed alkaline phosphatase activities in the cancer group by mean (SEM) of 17 (4) (p = 0.006) compared with no change in the other groups. Butyrate suppressed G:P ratios only in the cancer (6 (3)%, p = 0.03) and ulcerative colitis groups (5 (3)%, p = 0.04) and the changes in both were different (p < 0.05) from those in normal cells (increase of 10 (7)%). Changes in ulcerative colitis were different from those in Crohn's disease (p = 0.029). Responses were independent of the presence or absence of mucosal inflammation. These data confirm the diffuse nature of epithelial abnormalities in colorectal cancer. In ulcerative colitis, a different pattern of abnormality occurs, supporting the notion that the epithelium is also diffusely abnormal independent of mucosal inflammation.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allan A., Bristol J. B., Williamson R. C. Crypt cell production rate in ulcerative proctocolitis: differential increments in remission and relapse. Gut. 1985 Oct;26(10):999–1003. doi: 10.1136/gut.26.10.999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bleiberg H., Mainguet P., Galand P., Chretien J., Dupont-Mairesse N. Cell renewal in the human rectum. In vitro autoradiographic study on active ulcerative colitis. Gastroenterology. 1970 Jun;58(6):851–855. [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cooper H. S., Steplewski Z. Immunohistologic study of ulcerative colitis with monoclonal antibodies against tumor-associated and/or differentiation antigens. Gastroenterology. 1988 Sep;95(3):686–693. doi: 10.1016/s0016-5085(88)80015-5. [DOI] [PubMed] [Google Scholar]
- Delpre G., Avidor I., Steinherz R., Kadish U., Ben-Bassat M. Ultrastructural abnormalities in endoscopically and histologically normal and involved colon in ulcerative colitis. Am J Gastroenterol. 1989 Sep;84(9):1038–1046. [PubMed] [Google Scholar]
- Deschner E. E., Lipkin M. Proliferative patterns in colonic mucosa in familial polyposis. Cancer. 1975 Feb;35(2):413–418. doi: 10.1002/1097-0142(197502)35:2<413::aid-cncr2820350217>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L., Trier J. S. Epithelial cell renewal in cultured rectal biopsies in ulcerative colitis. Gastroenterology. 1973 Mar;64(3):383–390. [PubMed] [Google Scholar]
- Fairbairn L. J., Cowling G. J., Reipert B. M., Dexter T. M. Suppression of apoptosis allows differentiation and development of a multipotent hemopoietic cell line in the absence of added growth factors. Cell. 1993 Sep 10;74(5):823–832. doi: 10.1016/0092-8674(93)90462-y. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Vogelstein B. A genetic model for colorectal tumorigenesis. Cell. 1990 Jun 1;61(5):759–767. doi: 10.1016/0092-8674(90)90186-i. [DOI] [PubMed] [Google Scholar]
- Finnie I. A., Taylor B. A., Rhodes J. M. Ileal and colonic epithelial metabolism in quiescent ulcerative colitis: increased glutamine metabolism in distal colon but no defect in butyrate metabolism. Gut. 1993 Nov;34(11):1552–1558. doi: 10.1136/gut.34.11.1552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P. R., Folino M., Rosella O., Finch C. F., Moeller I., Alexeyeff M., Lindley J., Young G. P. Neoplasia and hyperplasia of large bowel: focal lesions in an abnormal epithelium. Gastroenterology. 1992 Nov;103(5):1452–1459. doi: 10.1016/0016-5085(92)91164-y. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Moeller I., Kagelari O., Folino M., Young G. P. Contrasting effects of butyrate on the expression of phenotypic markers of differentiation in neoplastic and non-neoplastic colonic epithelial cells in vitro. J Gastroenterol Hepatol. 1992 Mar-Apr;7(2):165–172. doi: 10.1111/j.1440-1746.1992.tb00956.x. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Pavli P. Pathogenic factors in inflammatory bowel disease. I. Ulcerative colitis. Dig Dis. 1992;10(1):17–28. doi: 10.1159/000171340. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., Rosella O., Rosella G., Young G. P. Butyrate is a potent inhibitor of urokinase secretion by normal colonic epithelium in vitro. Gastroenterology. 1994 Aug;107(2):410–419. doi: 10.1016/0016-5085(94)90166-x. [DOI] [PubMed] [Google Scholar]
- Gibson P. R., van de Pol E., Maxwell L. E., Gabriel A., Doe W. F. Isolation of colonic crypts that maintain structural and metabolic viability in vitro. Gastroenterology. 1989 Feb;96(2 Pt 1):283–291. doi: 10.1016/0016-5085(89)91549-7. [DOI] [PubMed] [Google Scholar]
- Gibson P., Rosella O., Rosella G., Young G. Secretion of urokinase and plasminogen activator inhibitor-1 by normal colonic epithelium in vitro. Gut. 1994 Jul;35(7):969–975. doi: 10.1136/gut.35.7.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P., Rosella O., Young G. Serum free medium increases expression of markers of differentiation in human colonic crypt cells. Gut. 1994 Jun;35(6):791–797. doi: 10.1136/gut.35.6.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenstein A. J., Panvelliwalla D. K., Katz L. B., Heimann T. M., Donnelly J., Pertsimlidis D., Geller S., Smith H., Aufses A. H., Jr Tissue carcinoembryonic antigen, dysplasia, and disease duration in colonic inflammatory bowel disease. Am J Gastroenterol. 1982 Apr;77(4):212–215. [PubMed] [Google Scholar]
- Haviland A. E., Borowitz M. J., Lan M. S., Kaufman B., Khorrami A., Phelps P. C., Metzgar R. S. Aberrant expression of monoclonal antibody-defined colonic mucosal antigens in inflammatory bowel disease. Gastroenterology. 1988 Nov;95(5):1302–1311. doi: 10.1016/0016-5085(88)90365-4. [DOI] [PubMed] [Google Scholar]
- Lee Y. S. Background mucosal changes in colorectal carcinomas. Cancer. 1988 Apr 15;61(8):1563–1570. [PubMed] [Google Scholar]
- McCall C. A., Cohen J. J. Programmed cell death in terminally differentiating keratinocytes: role of endogenous endonuclease. J Invest Dermatol. 1991 Jul;97(1):111–114. doi: 10.1111/1523-1747.ep12478519. [DOI] [PubMed] [Google Scholar]
- Morita H., Kettlewell M. G., Jewell D. P., Kent P. W. Glycosylation and sulphation of colonic mucus glycoproteins in patients with ulcerative colitis and in healthy subjects. Gut. 1993 Jul;34(7):926–932. doi: 10.1136/gut.34.7.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A. Alterations in mucosal content of colonic glycoconjugates in inflammatory bowel disease defined by monoclonal antibodies. Gastroenterology. 1988 Aug;95(2):379–387. doi: 10.1016/0016-5085(88)90494-5. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Fournier D. A. Emergence of antigenic glycoprotein structures in ulcerative colitis detected through monoclonal antibodies. Gastroenterology. 1988 Aug;95(2):371–378. doi: 10.1016/0016-5085(88)90493-3. [DOI] [PubMed] [Google Scholar]
- Podolsky D. K., Isselbacher K. J. Glycoprotein composition of colonic mucosa. Specific alterations in ulcerative colitis. Gastroenterology. 1984 Nov;87(5):991–998. [PubMed] [Google Scholar]
- Ponz de Leon M., Roncucci L., Di Donato P., Tassi L., Smerieri O., Amorico M. G., Malagoli G., De Maria D., Antonioli A., Chahin N. J. Pattern of epithelial cell proliferation in colorectal mucosa of normal subjects and of patients with adenomatous polyps or cancer of the large bowel. Cancer Res. 1988 Jul 15;48(14):4121–4126. [PubMed] [Google Scholar]
- Roediger W. E. The starved colon--diminished mucosal nutrition, diminished absorption, and colitis. Dis Colon Rectum. 1990 Oct;33(10):858–862. doi: 10.1007/BF02051922. [DOI] [PubMed] [Google Scholar]
- Serafini E. P., Kirk A. P., Chambers T. J. Rate and pattern of epithelial cell proliferation in ulcerative colitis. Gut. 1981 Aug;22(8):648–652. doi: 10.1136/gut.22.8.648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shamsuddin A. K., Weiss L., Phelps P. C., Trump B. F. Colon epithelium. IV. Human colon carcinogenesis. Changes in human colon mucosa adjacent to and remote from carcinomas of the colon. J Natl Cancer Inst. 1981 Feb;66(2):413–419. [PubMed] [Google Scholar]
- Terpstra O. T., van Blankenstein M., Dees J., Eilers G. A. Abnormal pattern of cell proliferation in the entire colonic mucosa of patients with colon adenoma or cancer. Gastroenterology. 1987 Mar;92(3):704–708. doi: 10.1016/0016-5085(87)90021-7. [DOI] [PubMed] [Google Scholar]
- Wilson R. G., Smith A. N., Bird C. C. Immunohistochemical detection of abnormal cell proliferation in colonic mucosa of subjects with polyps. J Clin Pathol. 1990 Sep;43(9):744–747. doi: 10.1136/jcp.43.9.744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf B. C., D'Emilia J. C., Salem R. R., DeCoste D., Sears H. F., Gottlieb L. S., Steele G. D., Jr Detection of the tumor-associated glycoprotein antigen (TAG-72) in premalignant lesions of the colon. J Natl Cancer Inst. 1989 Dec 20;81(24):1913–1917. doi: 10.1093/jnci/81.24.1913. [DOI] [PubMed] [Google Scholar]
- Young G. P., Rose I. S., Cropper S., Seetharam S., Alpers D. H. Hepatic clearance of rat plasma intestinal alkaline phosphatase. Am J Physiol. 1984 Oct;247(4 Pt 1):G419–G426. doi: 10.1152/ajpgi.1984.247.4.G419. [DOI] [PubMed] [Google Scholar]


