Abstract
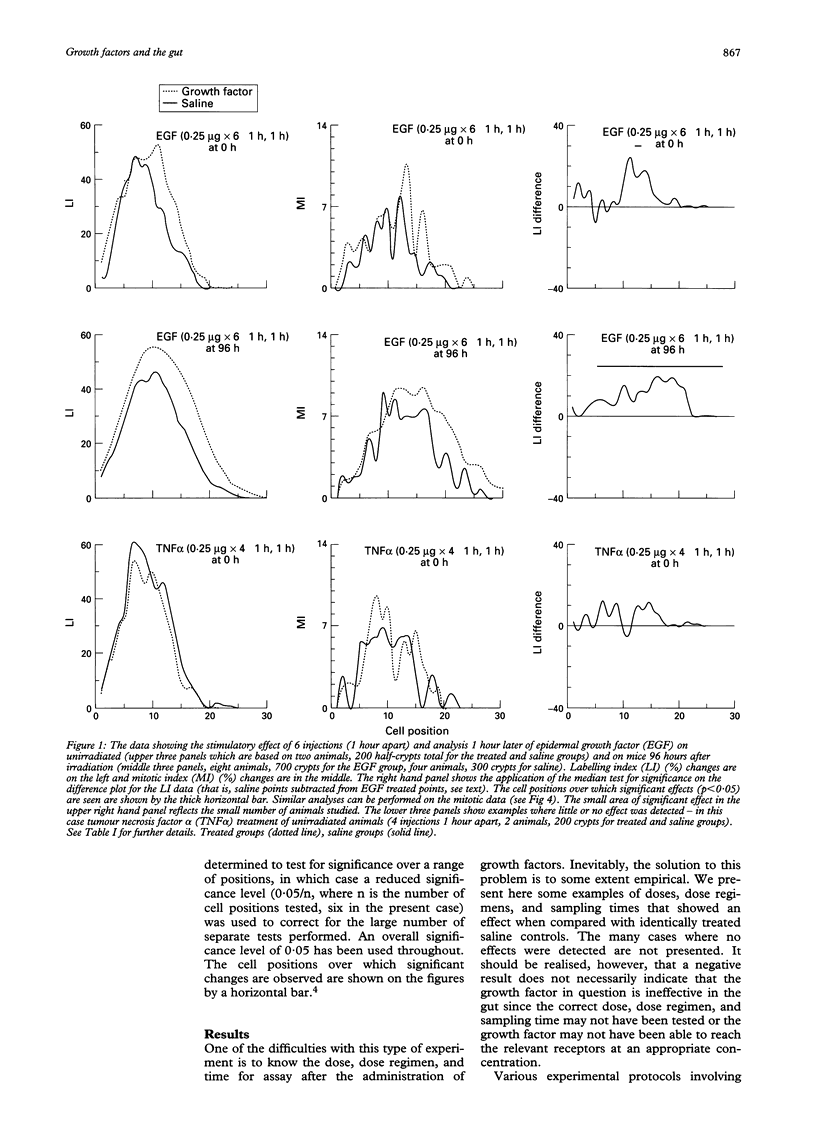
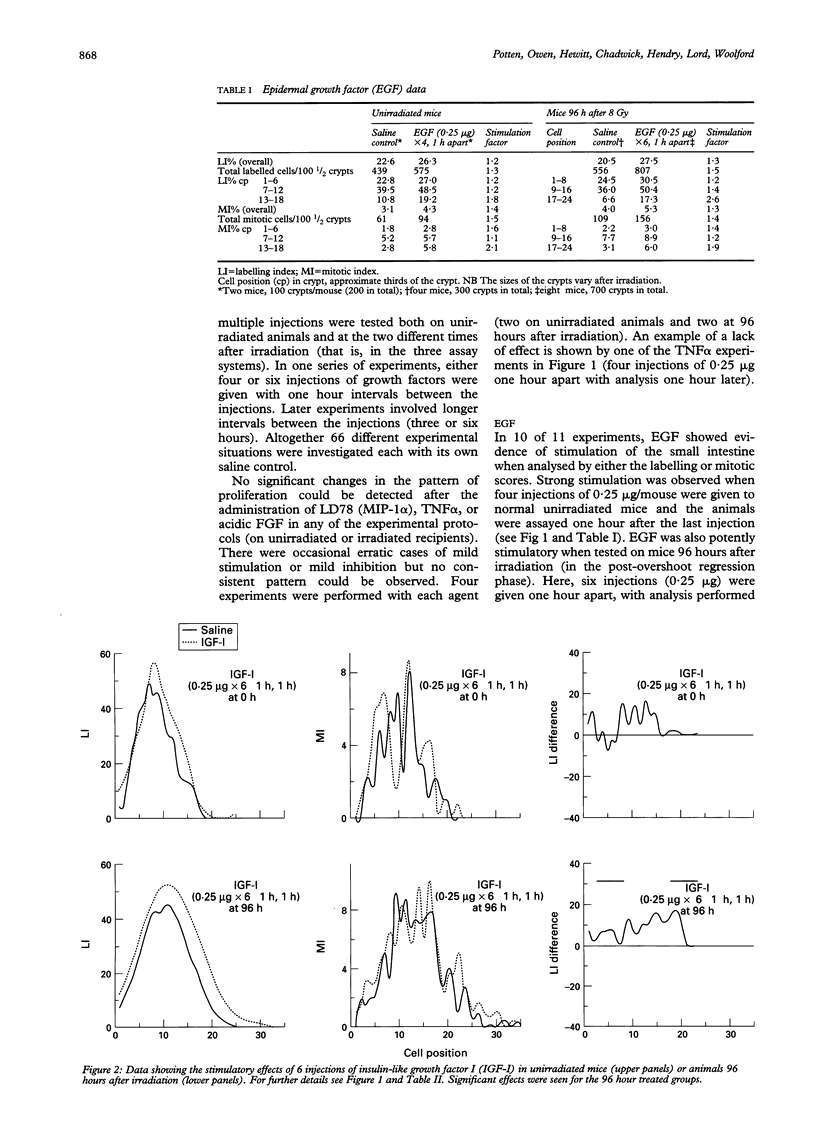
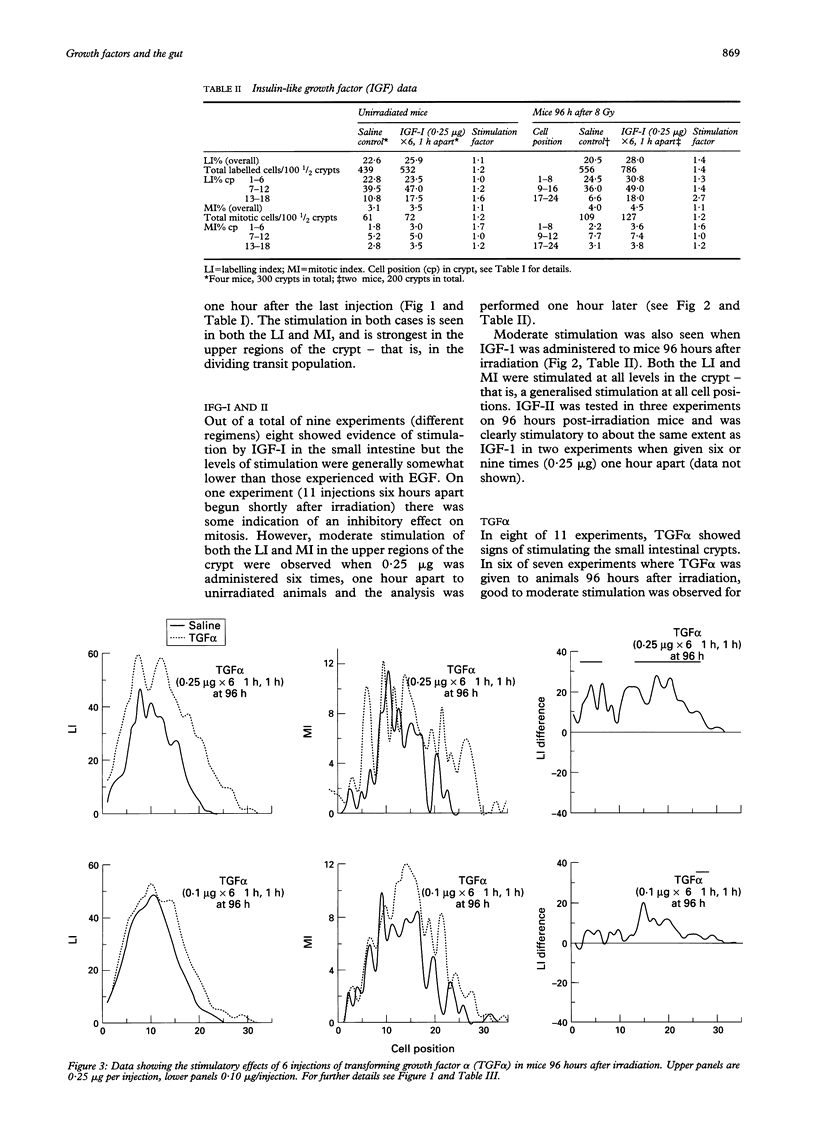
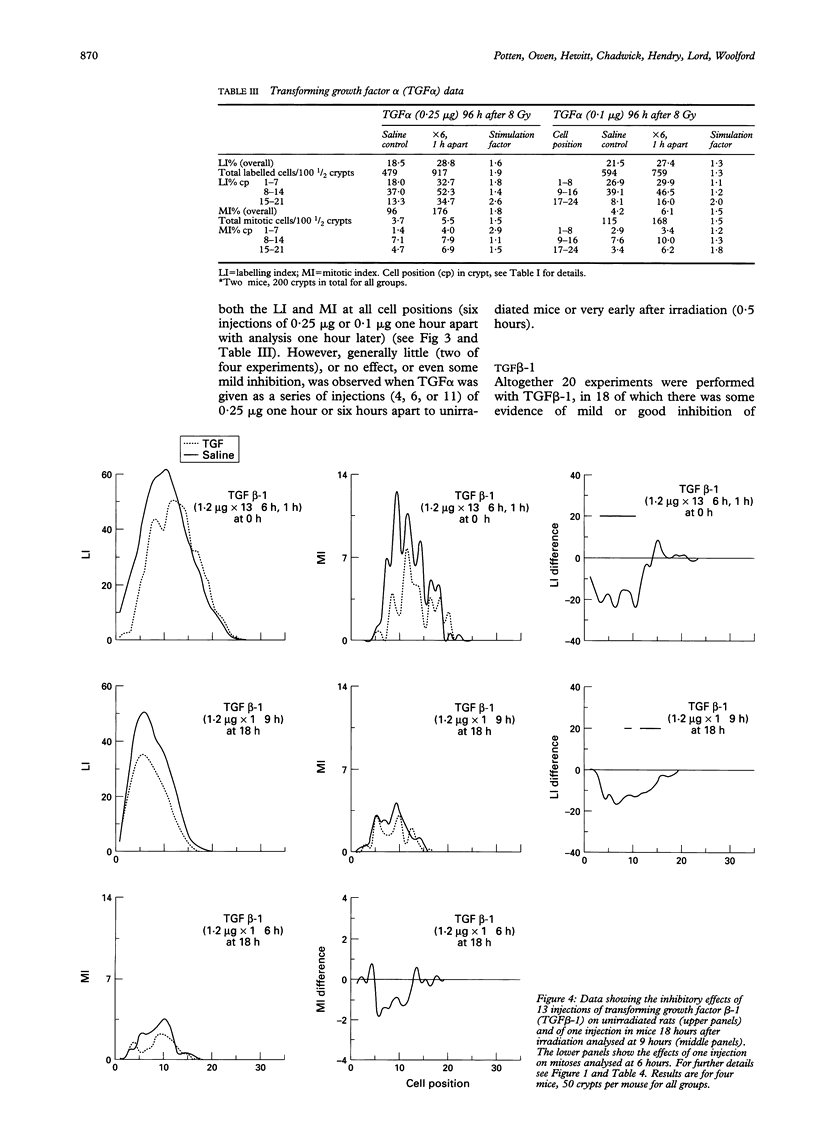
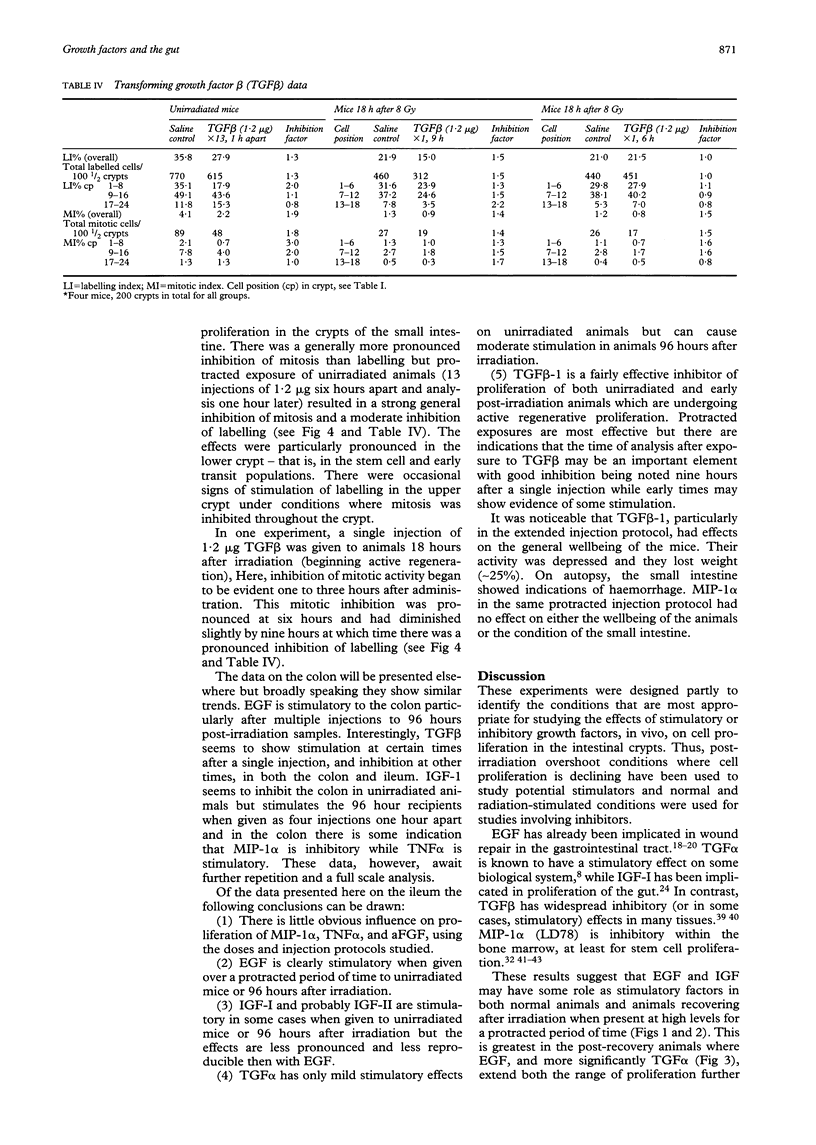
The effects of epidermal growth factor (EGF), transforming growth factor alpha (TGF alpha), insulin-like growth factor (IGF) I and II, acidic fibroblast growth factor (FGF), tumour necrosis factor alpha (TNF alpha), macrophage inhibitory protein 1 alpha (MIP-1 alpha) (LD78), and TGF beta-1 on cell proliferation in the crypts of the small intestine of mice were investigated. Various doses and dosing regimens were tested. Three in vivo assays were developed, in each case involving detailed cell positional analysis of methyl tritiated thymidine labelling and mitotic activity. These allowed deductions to be made about the regions of the crypt and hence regions of the proliferative hierarchy (stem cells versus dividing transit cells) that are affected by treatment with growth factors. The assays involved: (1) normal untreated mice (an assay most likely to be effective for detecting inhibitors); (2) mice shortly after whole body irradiation when compensatory proliferation has been endogenously triggered (another assay for inhibitory factors, possibly ones associated specifically with the regenerative process); and (3) mice at late times (96 hours) after irradiation in the regression phase after a proliferative overshoot (an assay designed to detect stimulators). Little effect was seen after treatment with acidic FGF, TNF alpha, or MIP-1 alpha but EGF, IGF-I and II, and TGF alpha can all be seen to exert some stimulatory effects on labelling or mitosis. EGF and IGF-I stimulate both unirradiated mice and 96 hour recipients, while TGF alpha had a greater effect on the 96 hour animals. In all cases, multiple doses were used. TGF beta-1 was an effective inhibitor of proliferation in unirradiated and early regenerating (18 hour) animals. EGF was the most effective of the stimulators, raising the levels of proliferation at all positions in the crypt, but particularly in the upper crypt. IGF-I also exerted its effect predominantly in the upper crypt, while TGF alpha raised proliferation at all cell positions. TGF beta-1 tended to have its strongest inhibitory effects in the lower (stem cell) regions of the crypt.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Avery A., Paraskeva C., Hall P., Flanders K. C., Sporn M., Moorghen M. TGF-beta expression in the human colon: differential immunostaining along crypt epithelium. Br J Cancer. 1993 Jul;68(1):137–139. doi: 10.1038/bjc.1993.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnard J. A., Beauchamp R. D., Coffey R. J., Moses H. L. Regulation of intestinal epithelial cell growth by transforming growth factor type beta. Proc Natl Acad Sci U S A. 1989 Mar;86(5):1578–1582. doi: 10.1073/pnas.86.5.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blay J., Brown K. D. Epidermal growth factor promotes the chemotactic migration of cultured rat intestinal epithelial cells. J Cell Physiol. 1985 Jul;124(1):107–112. doi: 10.1002/jcp.1041240117. [DOI] [PubMed] [Google Scholar]
- Cartlidge S. A., Elder J. B. Transforming growth factor alpha and epidermal growth factor levels in normal human gastrointestinal mucosa. Br J Cancer. 1989 Nov;60(5):657–660. doi: 10.1038/bjc.1989.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakrabarty S., Fan D., Varani J. Modulation of differentiation and proliferation in human colon carcinoma cells by transforming growth factor beta 1 and beta 2. Int J Cancer. 1990 Sep 15;46(3):493–499. doi: 10.1002/ijc.2910460328. [DOI] [PubMed] [Google Scholar]
- Conteas C. N., Majumdar A. P. The effects of gastrin, epidermal growth factor, and somatostatin on DNA synthesis in a small intestinal crypt cell line (IEC-6). Proc Soc Exp Biol Med. 1987 Mar;184(3):307–311. doi: 10.3181/00379727-184-42484. [DOI] [PubMed] [Google Scholar]
- Cove F. L., Evans G. S. The use of an intestinal epithelial cell line as a biological assay system of growth factor activity. Biochem Soc Trans. 1992 May;20(2):175S–175S. doi: 10.1042/bst020175s. [DOI] [PubMed] [Google Scholar]
- Derynck R. Transforming growth factor alpha. Cell. 1988 Aug 26;54(5):593–595. doi: 10.1016/s0092-8674(88)80001-1. [DOI] [PubMed] [Google Scholar]
- Dyduch A. Proliferative effects of epidermal growth factor on the intestinal epithelium of mice. Rom J Morphol Embryol. 1990 Apr-Jun;36(2):141–143. [PubMed] [Google Scholar]
- Goodlad R. A., Lee C. Y., Wright N. A. Cell proliferation in the small intestine and colon of intravenously fed rats: effects of urogastrone-epidermal growth factor. Cell Prolif. 1992 Sep;25(5):393–404. doi: 10.1111/j.1365-2184.1992.tb01449.x. [DOI] [PubMed] [Google Scholar]
- Graham G. J., Wright E. G., Hewick R., Wolpe S. D., Wilkie N. M., Donaldson D., Lorimore S., Pragnell I. B. Identification and characterization of an inhibitor of haemopoietic stem cell proliferation. Nature. 1990 Mar 29;344(6265):442–444. doi: 10.1038/344442a0. [DOI] [PubMed] [Google Scholar]
- Guo Y. S., Narayan S., Yallampalli C., Singh P. Characterization of insulinlike growth factor I receptors in human colon cancer. Gastroenterology. 1992 Apr;102(4 Pt 1):1101–1108. [PubMed] [Google Scholar]
- Kurokowa M., Lynch K., Podolsky D. K. Effects of growth factors on an intestinal epithelial cell line: transforming growth factor beta inhibits proliferation and stimulates differentiation. Biochem Biophys Res Commun. 1987 Feb 13;142(3):775–782. doi: 10.1016/0006-291x(87)91481-1. [DOI] [PubMed] [Google Scholar]
- Lahm H., Odartchenko N. Role of transforming growth factor beta in colorectal cancer. Growth Factors. 1993;9(1):1–9. doi: 10.3109/08977199308991577. [DOI] [PubMed] [Google Scholar]
- Li Y. Q., Fan C. Y., O'Connor P. J., Winton D. J., Potten C. S. Target cells for the cytotoxic effects of carcinogens in the murine small bowel. Carcinogenesis. 1992 Mar;13(3):361–368. doi: 10.1093/carcin/13.3.361. [DOI] [PubMed] [Google Scholar]
- Lord B. I., Dexter T. M., Clements J. M., Hunter M. A., Gearing A. J. Macrophage-inflammatory protein protects multipotent hematopoietic cells from the cytotoxic effects of hydroxyurea in vivo. Blood. 1992 May 15;79(10):2605–2609. [PubMed] [Google Scholar]
- Lord B. I., Mori K. J., Wright E. G., Lajtha L. G. Inhibitor of stem cell proliferation in normal bone marrow. Br J Haematol. 1976 Nov;34(3):441–445. doi: 10.1111/j.1365-2141.1976.tb03590.x. [DOI] [PubMed] [Google Scholar]
- Malo C., Ménard D. Influence of epidermal growth factor on the development of suckling mouse intestinal mucosa. Gastroenterology. 1982 Jul;83(1 Pt 1):28–35. [PubMed] [Google Scholar]
- Migdalska A., Molineux G., Demuynck H., Evans G. S., Ruscetti F., Dexter T. M. Growth inhibitory effects of transforming growth factor-beta 1 in vivo. Growth Factors. 1991;4(3):239–245. doi: 10.3109/08977199109104820. [DOI] [PubMed] [Google Scholar]
- Murthy U., Anzano M. A., Greig R. G. Expression of TGF-alpha/EGF and TGF-beta receptors in human colon carcinoma cell lines. Int J Cancer. 1989 Jul 15;44(1):110–115. doi: 10.1002/ijc.2910440120. [DOI] [PubMed] [Google Scholar]
- Park J. H., Vanderhoof J. A., Blackwood D., Macdonald R. G. Characterization of type I and type II insulin-like growth factor receptors in an intestinal epithelial cell line. Endocrinology. 1990 Jun;126(6):2998–3005. doi: 10.1210/endo-126-6-2998. [DOI] [PubMed] [Google Scholar]
- Parkinson E. K., Graham G. J., Daubersies P., Burns J. E., Heufler C., Plumb M., Schuler G., Pragnell I. B. Hemopoietic stem cell inhibitor (SCI/MIP-1 alpha) also inhibits clonogenic epidermal keratinocyte proliferation. J Invest Dermatol. 1993 Aug;101(2):113–117. doi: 10.1111/1523-1747.ep12363603. [DOI] [PubMed] [Google Scholar]
- Potten C. S. A comprehensive study of the radiobiological response of the murine (BDF1) small intestine. Int J Radiat Biol. 1990 Dec;58(6):925–973. doi: 10.1080/09553009014552281. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Chadwick C. A. Small intestinal growth regulatory factors extracted by simple diffusion from intact irradiated intestine and tested in vivo. Growth Factors. 1994;10(1):63–75. doi: 10.3109/08977199409019604. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Chwalinski S., Swindell R., Palmer M. The spatial organization of the hierarchical proliferative cells of the crypts of the small intestine into clusters of 'synchronized' cells. Cell Tissue Kinet. 1982 Jul;15(4):351–370. doi: 10.1111/j.1365-2184.1982.tb01053.x. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Kellett M., Roberts S. A., Rew D. A., Wilson G. D. Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut. 1992 Jan;33(1):71–78. doi: 10.1136/gut.33.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S., Loeffler M. Stem cells: attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990 Dec;110(4):1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Owen G., Roberts S. A. The temporal and spatial changes in cell proliferation within the irradiated crypts of the murine small intestine. Int J Radiat Biol. 1990 Jan;57(1):185–199. doi: 10.1080/09553009014550431. [DOI] [PubMed] [Google Scholar]
- Pérez-Tomás R., Culleré X., Díaz C. Immunohistochemical localization of transforming growth factor alpha in the developing rat colon. Gastroenterology. 1993 Mar;104(3):789–795. doi: 10.1016/0016-5085(93)91014-9. [DOI] [PubMed] [Google Scholar]
- Roberts A. B., Sporn M. B. Physiological actions and clinical applications of transforming growth factor-beta (TGF-beta). Growth Factors. 1993;8(1):1–9. doi: 10.3109/08977199309029129. [DOI] [PubMed] [Google Scholar]
- Sizeland A., Bol S., Burgess A. W. The action of epidermal growth factor (EGF) is limited to specific phases of the cell cycle in an EGF dependent colonic cell line. Growth Factors. 1991;4(2):129–143. doi: 10.3109/08977199109000264. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B. Transforming growth factor-beta: recent progress and new challenges. J Cell Biol. 1992 Dec;119(5):1017–1021. doi: 10.1083/jcb.119.5.1017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tejero C., Testa N. G., Lord B. I. The cellular specificity of haemopoietic stem cell proliferation regulators. Br J Cancer. 1984 Sep;50(3):335–341. doi: 10.1038/bjc.1984.180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J. S., Saxena S. K., Sharp J. G. Effect of the duration of infusion of urogastrone on intestinal regeneration in rabbits. Cell Tissue Kinet. 1989 Jul;22(4):303–309. doi: 10.1111/j.1365-2184.1989.tb00215.x. [DOI] [PubMed] [Google Scholar]
- Whithead R. H., Nice E. C., Lloyd C. J., James R., Burgess A. W. Detection of colonic growth factors using a human colonic carcinoma cell line (LIM1215). Int J Cancer. 1990 Nov 15;46(5):858–863. doi: 10.1002/ijc.2910460518. [DOI] [PubMed] [Google Scholar]
- Wright N. A., Pike C., Elia G. Induction of a novel epidermal growth factor-secreting cell lineage by mucosal ulceration in human gastrointestinal stem cells. Nature. 1990 Jan 4;343(6253):82–85. doi: 10.1038/343082a0. [DOI] [PubMed] [Google Scholar]


