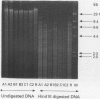
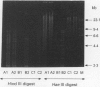
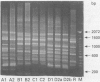
Abstract
The aim of this study was to find out if reinfection or recrudescence accounted for the recurrence of Helicobacter pylori infections after apparent eradication of the bacterium. Three hundred and twenty patients were treated with colloidal bismuth subcitrate (120 mg four times daily for four weeks), metronidazole and tetracycline (400 mg and 500 mg, respectively, thrice daily for the first week). H pylori was eradicated four weeks after the end of treatment as assessed by the rapid urease test, histological examination, Gram staining, and culture. However, the infection recurred in 29 (9.1%) of the patients one year after apparent eradication. Pre and posteradication isolates from five patients were available. DNA was extracted and used for restriction endonuclease analysis with Hind III and Hae III, and for polymerase chain reaction (PCR) based randomly amplified polymorphic DNA fingerprinting with a combination of two 10 nucleotide primers. Sodium dodecyl sulphate polyacrylamide gel electrophoretic analysis was performed also. Randomly amplified polymorphic DNA fingerprinting was unique in that it yielded highly discriminatory fingerprints, which showed that the pretreatment and recurrent isolates obtained from each of the five patients were indistinguishable from one another. This shows that recurrence of H pylori infection is probably caused by recrudescence and that the discriminatory power of randomly amplified polymorphic DNA fingerprinting is a practicable and discriminatory typing scheme for H pylori.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akopyanz N., Bukanov N. O., Westblom T. U., Kresovich S., Berg D. E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992 Oct 11;20(19):5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. D., Powell K. U., Burridge S. M., Harrison G., Rameh B., Weil J., Gant P. W., Jones P. H., Trowell J. E. Reinfection or recrudescence after apparently successful eradication of Helicobacter pylori infection: implications for treatment of patients with duodenal ulcer disease. Q J Med. 1993 Jun;86(6):375–382. [PubMed] [Google Scholar]
- Borody T., Andrews P., Mancuso N., Jankiewicz E., Brandl S. Helicobacter pylori reinfection 4 years post-eradication. Lancet. 1992 May 23;339(8804):1295–1295. doi: 10.1016/0140-6736(92)91622-f. [DOI] [PubMed] [Google Scholar]
- Costas M., Owen R. J., Bickley J., Morgan D. R. Molecular techniques for studying the epidemiology of infection by Helicobacter pylori. Scand J Gastroenterol Suppl. 1991;181:20–32. doi: 10.3109/00365529109093204. [DOI] [PubMed] [Google Scholar]
- Forbes G. M., Glaser M. E., Cullen D. J., Warren J. R., Christiansen K. J., Marshall B. J., Collins B. J. Duodenal ulcer treated with Helicobacter pylori eradication: seven-year follow-up. Lancet. 1994 Jan 29;343(8892):258–260. doi: 10.1016/s0140-6736(94)91111-8. [DOI] [PubMed] [Google Scholar]
- Goodwin C. S., Armstrong J. A., Marshall B. J. Campylobacter pyloridis, gastritis, and peptic ulceration. J Clin Pathol. 1986 Apr;39(4):353–365. doi: 10.1136/jcp.39.4.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham D. Y., Hepps K. S., Ramirez F. C., Lew G. M., Saeed Z. A. Treatment of Helicobacter pylori reduces the rate of rebleeding in peptic ulcer disease. Scand J Gastroenterol. 1993 Nov;28(11):939–942. doi: 10.3109/00365529309098288. [DOI] [PubMed] [Google Scholar]
- Husson M. O., Gottrand F., Turck D., Leclerc H. Detection of H. pylori in saliva using a monoclonal antibody. Zentralbl Bakteriol. 1993 Nov;279(4):466–471. doi: 10.1016/s0934-8840(11)80418-4. [DOI] [PubMed] [Google Scholar]
- Labenz J., Börsch G. Role of Helicobacter pylori eradication in the prevention of peptic ulcer bleeding relapse. Digestion. 1994;55(1):19–23. doi: 10.1159/000201117. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Langenberg W., Rauws E. A., Widjojokusumo A., Tytgat G. N., Zanen H. C. Identification of Campylobacter pyloridis isolates by restriction endonuclease DNA analysis. J Clin Microbiol. 1986 Sep;24(3):414–417. doi: 10.1128/jcm.24.3.414-417.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majewski S. I., Goodwin C. S. Restriction endonuclease analysis of the genome of Campylobacter pylori with a rapid extraction method: evidence for considerable genomic variation. J Infect Dis. 1988 Mar;157(3):465–471. doi: 10.1093/infdis/157.3.465. [DOI] [PubMed] [Google Scholar]
- Markwell M. A., Haas S. M., Bieber L. L., Tolbert N. E. A modification of the Lowry procedure to simplify protein determination in membrane and lipoprotein samples. Anal Biochem. 1978 Jun 15;87(1):206–210. doi: 10.1016/0003-2697(78)90586-9. [DOI] [PubMed] [Google Scholar]
- Mazurier S., van de Giessen A., Heuvelman K., Wernars K. RAPD analysis of Campylobacter isolates: DNA fingerprinting without the need to purify DNA. Lett Appl Microbiol. 1992 Jun;14(6):260–262. doi: 10.1111/j.1472-765x.1992.tb00700.x. [DOI] [PubMed] [Google Scholar]
- Nagata K., Satoh H., Iwahi T., Shimoyama T., Tamura T. Potent inhibitory action of the gastric proton pump inhibitor lansoprazole against urease activity of Helicobacter pylori: unique action selective for H. pylori cells. Antimicrob Agents Chemother. 1993 Apr;37(4):769–774. doi: 10.1128/aac.37.4.769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patchett S., Beattie S., Leen E., Keane C., O'Morain C. Helicobacter pylori and duodenal ulcer recurrence. Am J Gastroenterol. 1992 Jan;87(1):24–27. [PubMed] [Google Scholar]
- Rauws E. A., Tytgat G. N. Cure of duodenal ulcer associated with eradication of Helicobacter pylori. Lancet. 1990 May 26;335(8700):1233–1235. doi: 10.1016/0140-6736(90)91301-p. [DOI] [PubMed] [Google Scholar]
- Simor A. E., Shames B., Drumm B., Sherman P., Low D. E., Penner J. L. Typing of Campylobacter pylori by bacterial DNA restriction endonuclease analysis and determination of plasmid profile. J Clin Microbiol. 1990 Jan;28(1):83–86. doi: 10.1128/jcm.28.1.83-86.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tee W., Lambert J., Smallwood R., Schembri M., Ross B. C., Dwyer B. Ribotyping of Helicobacter pylori from clinical specimens. J Clin Microbiol. 1992 Jun;30(6):1562–1567. doi: 10.1128/jcm.30.6.1562-1567.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas J. E., Gibson G. R., Darboe M. K., Dale A., Weaver L. T. Isolation of Helicobacter pylori from human faeces. Lancet. 1992 Nov 14;340(8829):1194–1195. doi: 10.1016/0140-6736(92)92894-l. [DOI] [PubMed] [Google Scholar]
- Welsh J., McClelland M. Fingerprinting genomes using PCR with arbitrary primers. Nucleic Acids Res. 1990 Dec 25;18(24):7213–7218. doi: 10.1093/nar/18.24.7213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H. X., English L., Keane C. T., O'Morain C. A. Enhanced cultivation of Helicobacter pylori in liquid media. J Clin Pathol. 1993 Aug;46(8):750–753. doi: 10.1136/jcp.46.8.750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xia H. X., Keane C. T., O'Morain C. A. Pre-formed urease activity of Helicobacter pylori as determined by a viable cell count technique--clinical implications. J Med Microbiol. 1994 Jun;40(6):435–439. doi: 10.1099/00222615-40-6-435. [DOI] [PubMed] [Google Scholar]