Abstract
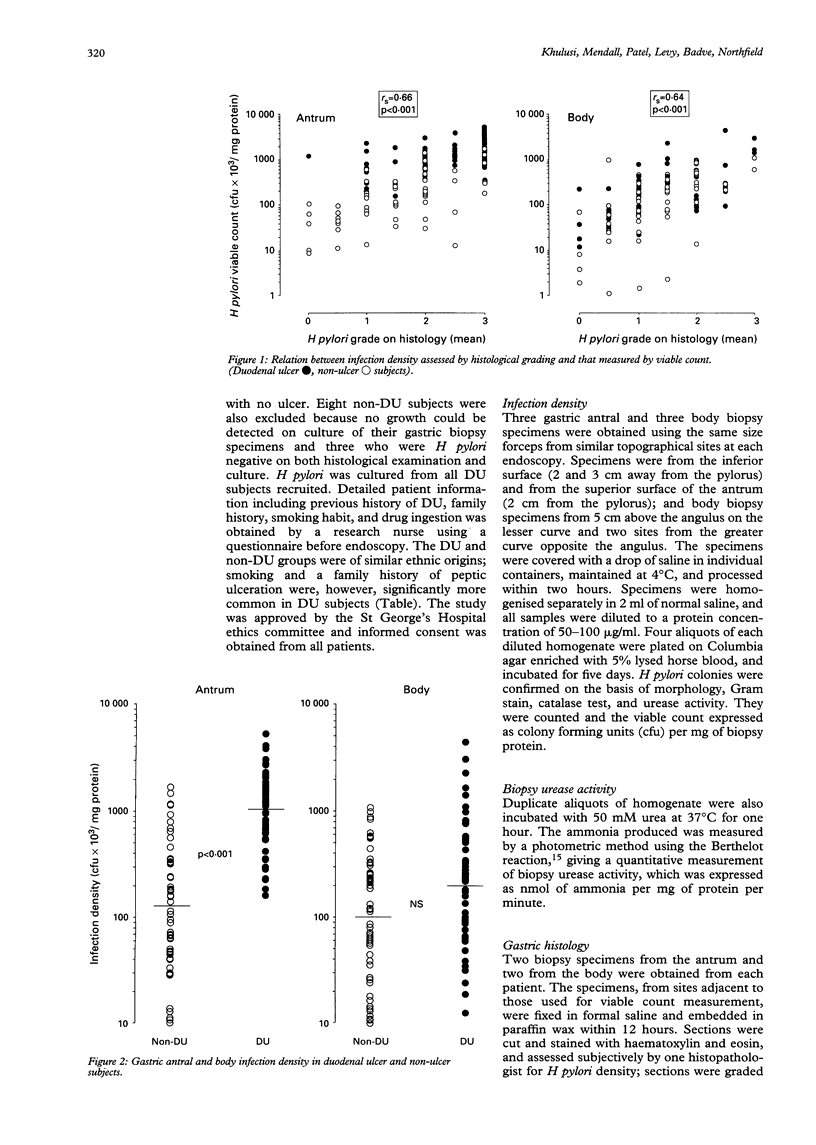
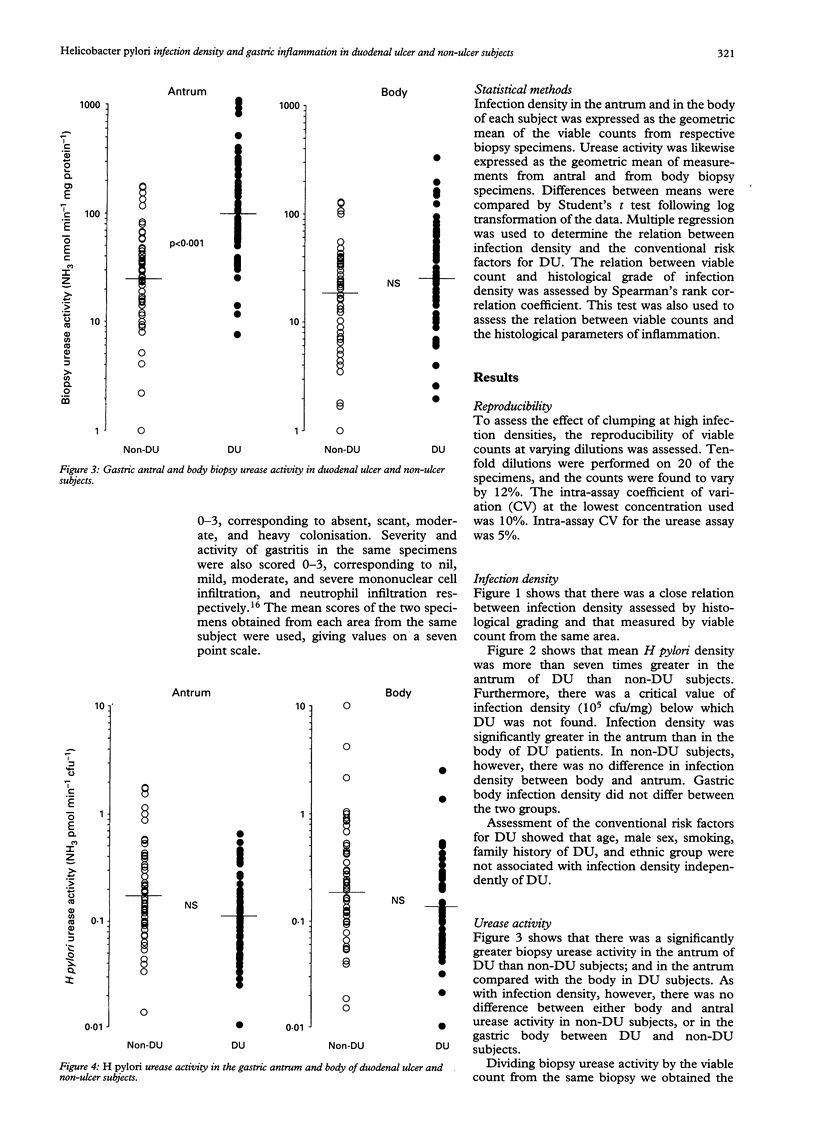
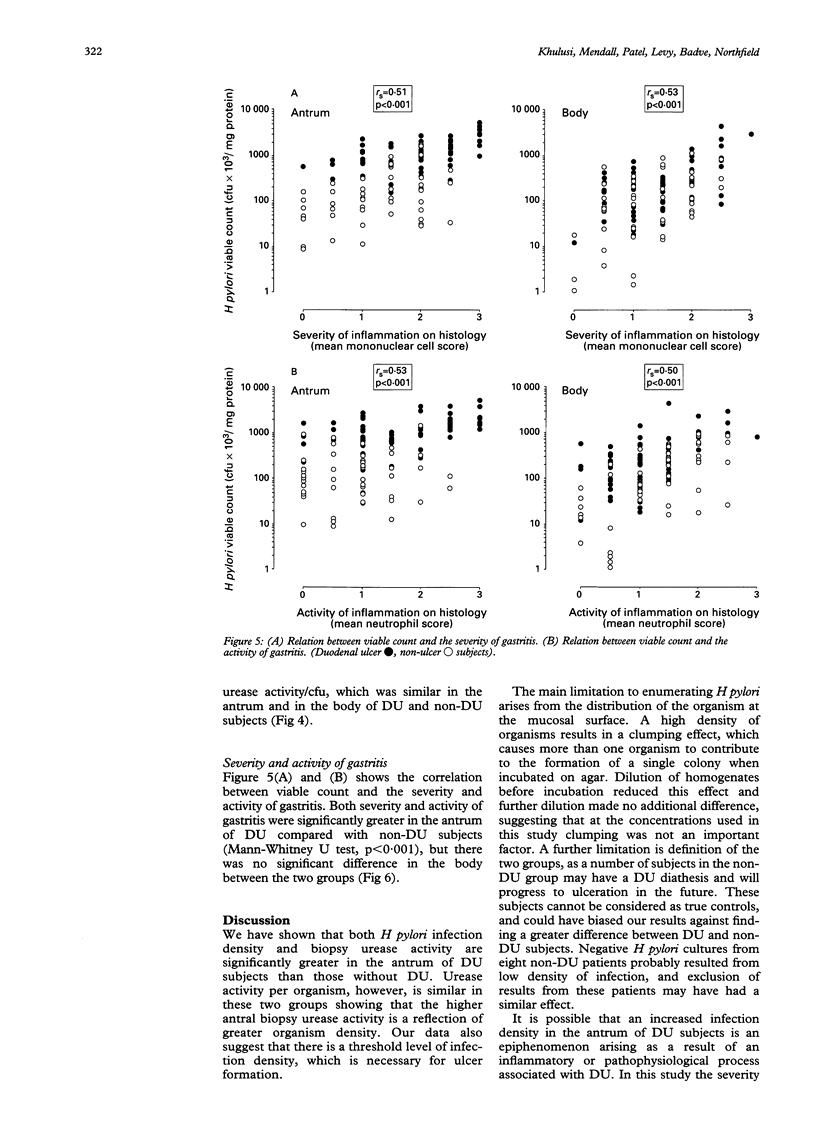
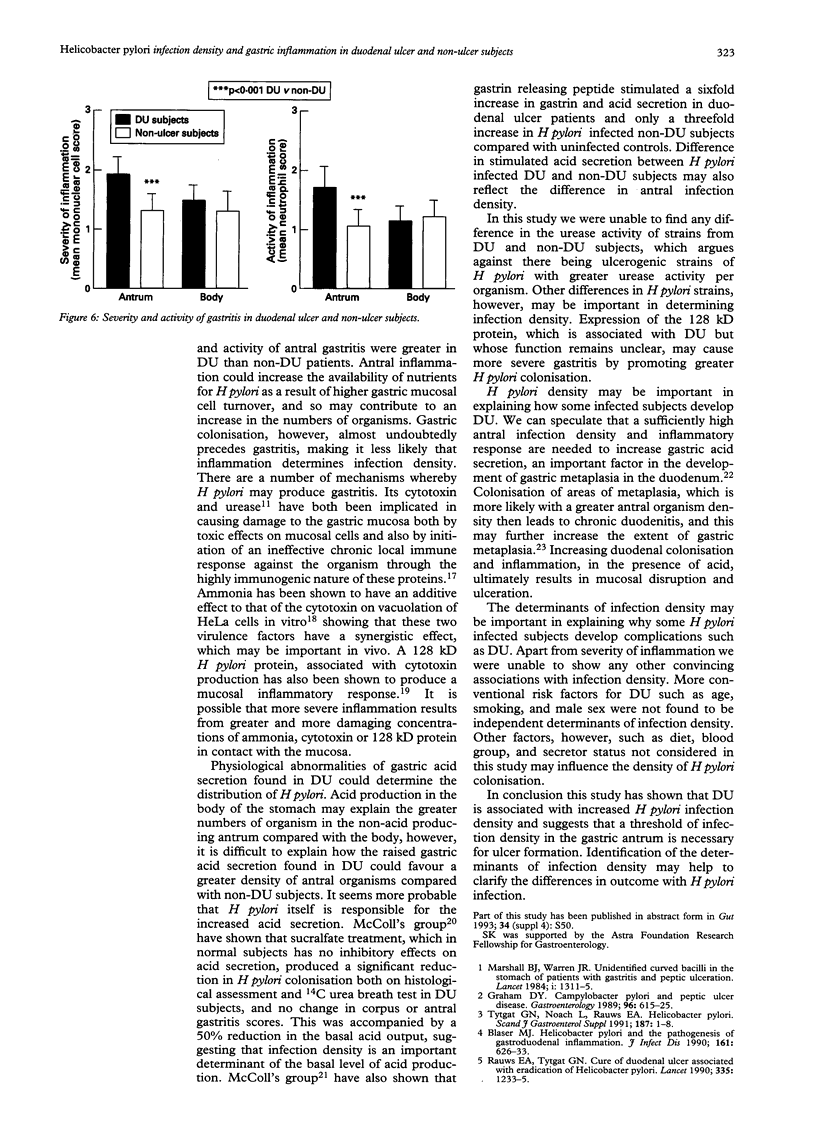
The factors that determine which Helicobacter pylori infected subjects develop duodenal ulcer (DU) are unclear. This study tested the hypothesis that infection density and urease activity are higher in DU than non-DU subjects. Fifty five DU and 55 age and sex matched non-DU subjects were studied. Quantitative methods were used for measuring infection density (viable organism count) and urease activity (Berthelot reaction). DU subjects had a greater antral infection density (geometric mean of colony forming units/mg biopsy protein; 10.5 x 10(5) v 1.3 x 10(5), p < 0.001). They also had higher biopsy urease activity (geometric mean of NH3 nmol/min-1/mg protein-1; 103 v 25, p < 0.001). Urease activity per organism, however, was similar in the two groups showing that high antral urease activity in DU was a reflection of organism density. DU was not present in subjects with an antral infection density less than 10(5) colony forming units/mg protein. A correlation was present between H pylori viable counts and the severity and activity of gastritis. Both severity and activity of gastritis were greater in the antrum of DU compared with non-DU subjects but there was no difference in the body between the two groups. It is concluded that antral H pylori infection density is probably an important determinant of DU development, and that there is a baseline of infection density that is necessary for ulcer formation.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beaney R. P., Lammertsma A. A., Jones T., McKenzie C. G., Halnan K. E. Positron emission tomography for in-vivo measurement of regional blood flow, oxygen utilisation, and blood volume in patients with breast carcinoma. Lancet. 1984 Jan 21;1(8369):131–134. doi: 10.1016/s0140-6736(84)90063-1. [DOI] [PubMed] [Google Scholar]
- Blaser M. J. Helicobacter pylori and the pathogenesis of gastroduodenal inflammation. J Infect Dis. 1990 Apr;161(4):626–633. doi: 10.1093/infdis/161.4.626. [DOI] [PubMed] [Google Scholar]
- Covacci A., Censini S., Bugnoli M., Petracca R., Burroni D., Macchia G., Massone A., Papini E., Xiang Z., Figura N. Molecular characterization of the 128-kDa immunodominant antigen of Helicobacter pylori associated with cytotoxicity and duodenal ulcer. Proc Natl Acad Sci U S A. 1993 Jun 15;90(12):5791–5795. doi: 10.1073/pnas.90.12.5791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Dooley C. P., Blaser M. J. Characterization of and human serologic response to proteins in Helicobacter pylori broth culture supernatants with vacuolizing cytotoxin activity. Infect Immun. 1990 Mar;58(3):603–610. doi: 10.1128/iai.58.3.603-610.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cover T. L., Puryear W., Perez-Perez G. I., Blaser M. J. Effect of urease on HeLa cell vacuolation induced by Helicobacter pylori cytotoxin. Infect Immun. 1991 Apr;59(4):1264–1270. doi: 10.1128/iai.59.4.1264-1270.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Shallcross T. M., Wyatt J. I., Taylor J. D., Heatley R. V., Rathbone B. J., Losowsky M. S. Mucosal humoral immune response to Helicobacter pylori in patients with duodenitis. Dig Dis Sci. 1991 Sep;36(9):1266–1273. doi: 10.1007/BF01307520. [DOI] [PubMed] [Google Scholar]
- Crabtree J. E., Taylor J. D., Wyatt J. I., Heatley R. V., Shallcross T. M., Tompkins D. S., Rathbone B. J. Mucosal IgA recognition of Helicobacter pylori 120 kDa protein, peptic ulceration, and gastric pathology. Lancet. 1991 Aug 10;338(8763):332–335. doi: 10.1016/0140-6736(91)90477-7. [DOI] [PubMed] [Google Scholar]
- Eaton K. A., Brooks C. L., Morgan D. R., Krakowka S. Essential role of urease in pathogenesis of gastritis induced by Helicobacter pylori in gnotobiotic piglets. Infect Immun. 1991 Jul;59(7):2470–2475. doi: 10.1128/iai.59.7.2470-2475.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgibbons P. L., Dooley C. P., Cohen H., Appleman M. D. Prevalence of gastric metaplasia, inflammation, and Campylobacter pylori in the duodenum of members of a normal population. Am J Clin Pathol. 1988 Dec;90(6):711–714. doi: 10.1093/ajcp/90.6.711. [DOI] [PubMed] [Google Scholar]
- Graham D. Y. Campylobacter pylori and peptic ulcer disease. Gastroenterology. 1989 Feb;96(2 Pt 2 Suppl):615–625. doi: 10.1016/s0016-5085(89)80057-5. [DOI] [PubMed] [Google Scholar]
- Khulusi S., Mendall M. A., Badve S., Patel P., Finlayson C., Northfield T. C. Effect of Helicobacter pylori eradication on gastric metaplasia of the duodenum. Gut. 1995 Feb;36(2):193–197. doi: 10.1136/gut.36.2.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leunk R. D., Johnson P. T., David B. C., Kraft W. G., Morgan D. R. Cytotoxic activity in broth-culture filtrates of Campylobacter pylori. J Med Microbiol. 1988 Jun;26(2):93–99. doi: 10.1099/00222615-26-2-93. [DOI] [PubMed] [Google Scholar]
- Mégraud F., Neman-Simha V., Brügmann D. Further evidence of the toxic effect of ammonia produced by Helicobacter pylori urease on human epithelial cells. Infect Immun. 1992 May;60(5):1858–1863. doi: 10.1128/iai.60.5.1858-1863.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scheppach W. Effects of short chain fatty acids on gut morphology and function. Gut. 1994 Jan;35(1 Suppl):S35–S38. doi: 10.1136/gut.35.1_suppl.s35. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tytgat G. N., Noach L., Rauws E. A. Helicobacter pylori. Scand J Gastroenterol Suppl. 1991;187:1–8. doi: 10.3109/00365529109098219. [DOI] [PubMed] [Google Scholar]
- Weiner A. J., Kuo G., Bradley D. W., Bonino F., Saracco G., Lee C., Rosenblatt J., Choo Q. L., Houghton M. Detection of hepatitis C viral sequences in non-A, non-B hepatitis. Lancet. 1990 Jan 6;335(8680):1–3. doi: 10.1016/0140-6736(90)90134-q. [DOI] [PubMed] [Google Scholar]
- Wyatt J. I., Rathbone B. J., Dixon M. F., Heatley R. V. Campylobacter pyloridis and acid induced gastric metaplasia in the pathogenesis of duodenitis. J Clin Pathol. 1987 Aug;40(8):841–848. doi: 10.1136/jcp.40.8.841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J. K., Goodwin C. S., Cooper M., Robinson J. Intracellular vacuolization caused by the urease of Helicobacter pylori. J Infect Dis. 1990 Jun;161(6):1302–1304. doi: 10.1093/infdis/161.6.1302. [DOI] [PubMed] [Google Scholar]



