Abstract
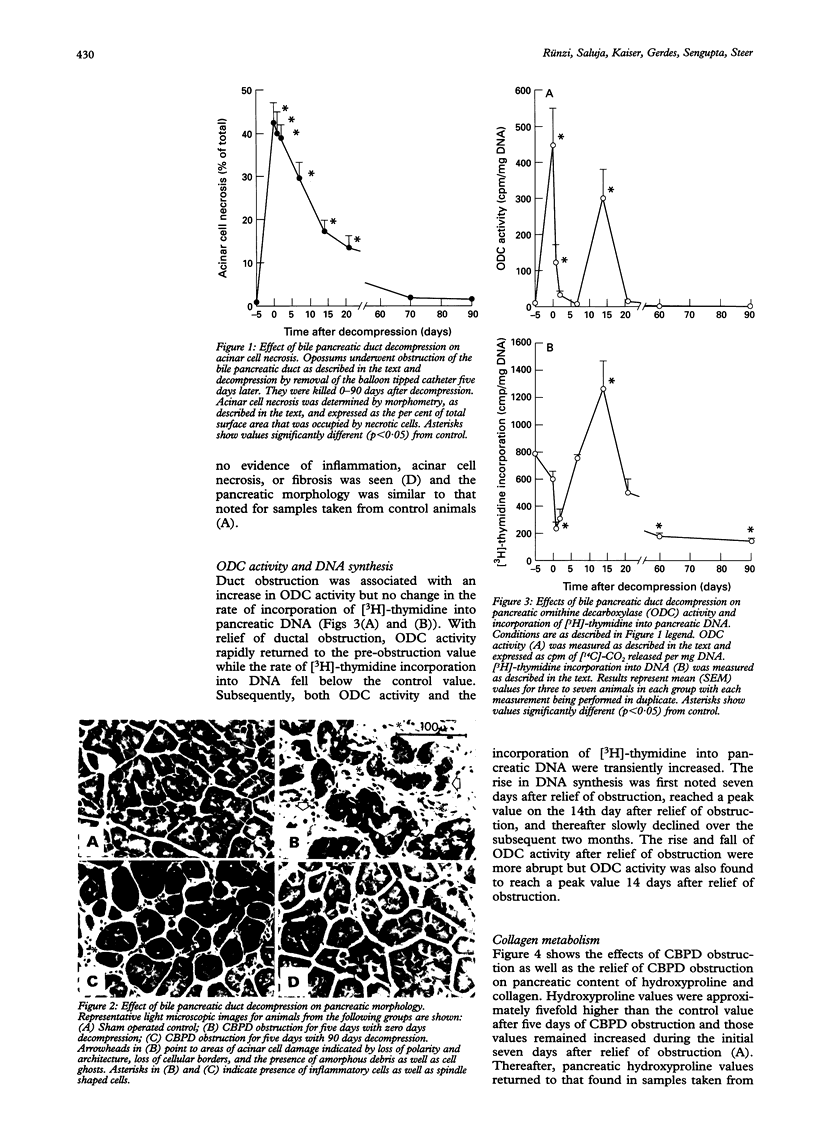
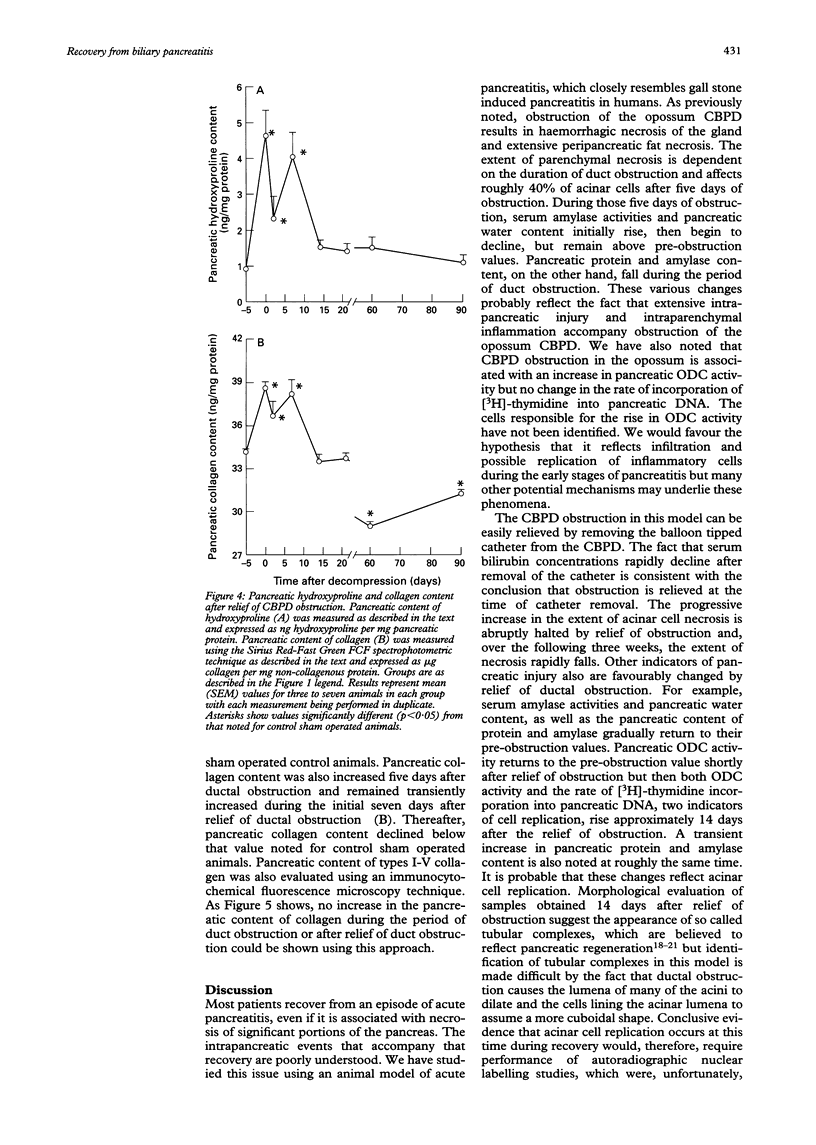
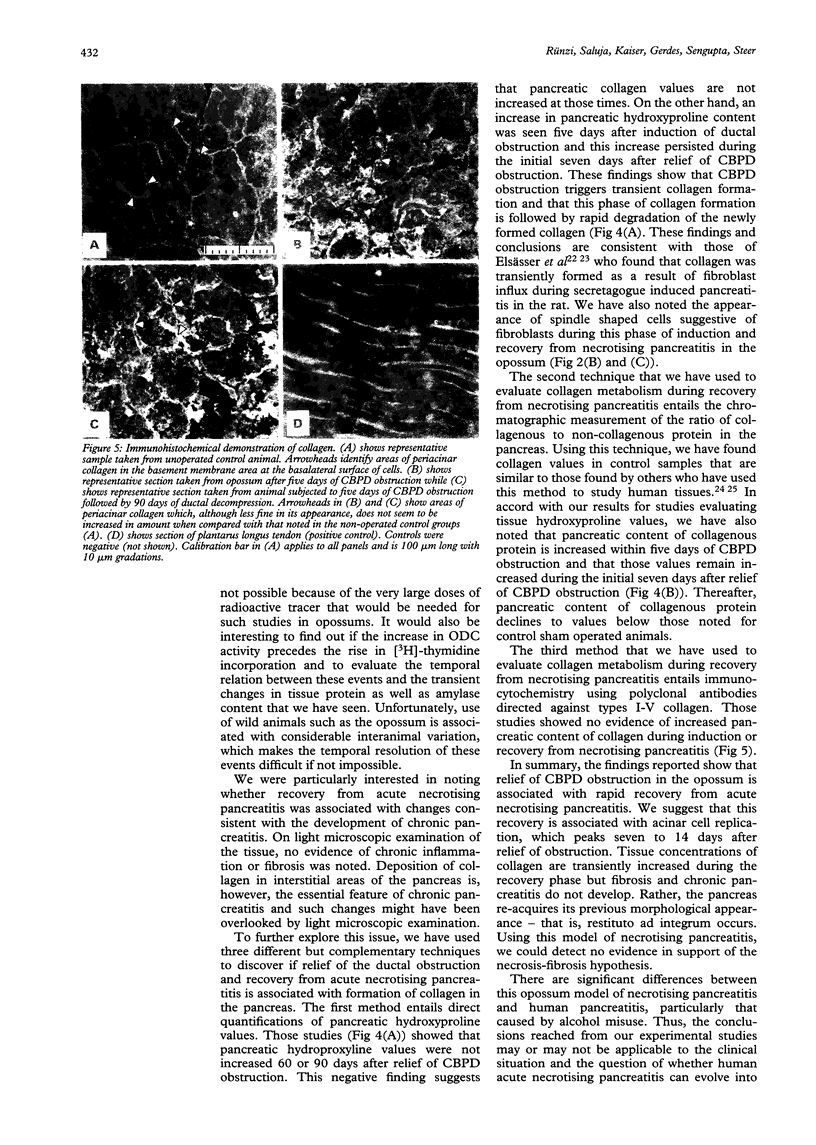
The events that characterise recovery from severe biliary pancreatitis have not been defined. This study used a reversible model of necrotising pancreatitis, induced by obstructing the opossum common bile pancreatic duct (CBPD), to evaluate this phenomenon. The CBPD of opossums was obstructed with a balloon tipped catheter for five days and then decompressed by removal of the catheter. Recovery was evaluated 0-90 days after relief of obstruction. Serum bilirubin and amylase values rapidly declined, reaching control values 7-14 days after removal of the obstructing catheter. Pancreatic protein and amylase values were transiently increased shortly after relief of obstruction but returned to control values 21 days after decompression. Pancreatic ornithine decarboxylase activity and incorporation of [3H]-thymidine into DNA were transiently increased 14 days after duct decompression suggesting that regeneration occurs at approximately that time. Foci of pancreatic necrosis involved roughly 40% of the gland at time of decompression but these foci gradually disappeared and the gland resembled that of control animals 60 days after decompression. Evidence of fibrosis or collagen deposition in the pancreas was not noted at any time. These studies show that recovery after necrotising biliary pancreatitis occurs comparatively rapidly and the restitution ad integrum occurs. Recovery from necrotising acute pancreatitis in this model is not associated with the development of chronic pancreatitis.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Angelini G., Cavallini G., Pederzoli P., Bovo P., Bassi C., Di Francesco V., Frulloni L., Sgarbi D., Talamini G., Castagnini A. Long-term outcome of acute pancreatitis: a prospective study with 118 patients. Digestion. 1993;54(3):143–147. doi: 10.1159/000201028. [DOI] [PubMed] [Google Scholar]
- Bedossa P., Lemaigre G., Bacci J., Martin E. Quantitative estimation of the collagen content in normal and pathologic pancreas tissue. Digestion. 1989;44(1):7–13. doi: 10.1159/000199886. [DOI] [PubMed] [Google Scholar]
- Dawra R., Saluja A., Lerch M. M., Saluja M., Logsdon C., Steer M. Stimulation of pancreatic growth by cholecystokinin is mediated by high affinity receptors on rat pancreatic acinar cells. Biochem Biophys Res Commun. 1993 Jun 30;193(3):814–820. doi: 10.1006/bbrc.1993.1698. [DOI] [PubMed] [Google Scholar]
- Elsässer H. P., Adler G., Kern H. F. Time course and cellular source of pancreatic regeneration following acute pancreatitis in the rat. Pancreas. 1986;1(5):421–429. doi: 10.1097/00006676-198609000-00006. [DOI] [PubMed] [Google Scholar]
- Elsässer H. P., Haake T., Grimmig M., Adler G., Kern H. F. Repetitive cerulein-induced pancreatitis and pancreatic fibrosis in the rat. Pancreas. 1992;7(3):385–390. doi: 10.1097/00006676-199205000-00017. [DOI] [PubMed] [Google Scholar]
- Ho K. C., Pang C. P. Automated analysis of urinary hydroxyproline. Clin Chim Acta. 1989 Nov;185(2):191–195. doi: 10.1016/0009-8981(89)90042-9. [DOI] [PubMed] [Google Scholar]
- Iovanna J. L., Odaira C., Berger Z., Sarles H. Temporary pseudochronic lesions during the recovery of acute necrohemorrhagic pancreatitis in rabbits. Pancreas. 1988;3(4):433–438. doi: 10.1097/00006676-198808000-00011. [DOI] [PubMed] [Google Scholar]
- Klöppel G., Maillet B. Pseudocysts in chronic pancreatitis: a morphological analysis of 57 resection specimens and 9 autopsy pancreata. Pancreas. 1991 May;6(3):266–274. [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Labarca C., Paigen K. A simple, rapid, and sensitive DNA assay procedure. Anal Biochem. 1980 Mar 1;102(2):344–352. doi: 10.1016/0003-2697(80)90165-7. [DOI] [PubMed] [Google Scholar]
- Langlois A., Morisset J. Effects of feeding, fasting, and caerulein treatment on ornithine decarboxylase in rat pancreas. Pancreas. 1991 Sep;6(5):534–541. doi: 10.1097/00006676-199109000-00006. [DOI] [PubMed] [Google Scholar]
- Lechene de la Porte P., Iovanna J., Odaira C., Choux R., Sarles H., Berger Z. Involvement of tubular complexes in pancreatic regeneration after acute necrohemorrhagic pancreatitis. Pancreas. 1991 May;6(3):298–306. doi: 10.1097/00006676-199105000-00007. [DOI] [PubMed] [Google Scholar]
- Lerch M. M., Saluja A. K., Dawra R., Ramaraò P., Saluja M., Steer M. L. Acute necrotizing pancreatitis in the opossum: earliest morphological changes involve acinar cells. Gastroenterology. 1992 Jul;103(1):205–213. doi: 10.1016/0016-5085(92)91114-j. [DOI] [PubMed] [Google Scholar]
- Lerch M. M., Saluja A. K., Rünzi M., Dawra R., Saluja M., Steer M. L. Pancreatic duct obstruction triggers acute necrotizing pancreatitis in the opossum. Gastroenterology. 1993 Mar;104(3):853–861. doi: 10.1016/0016-5085(93)91022-a. [DOI] [PubMed] [Google Scholar]
- López-De León A., Rojkind M. A simple micromethod for collagen and total protein determination in formalin-fixed paraffin-embedded sections. J Histochem Cytochem. 1985 Aug;33(8):737–743. doi: 10.1177/33.8.2410480. [DOI] [PubMed] [Google Scholar]
- Rünzi M., Saluja A., Lerch M. M., Dawra R., Nishino H., Steer M. L. Early ductal decompression prevents the progression of biliary pancreatitis: an experimental study in the opossum. Gastroenterology. 1993 Jul;105(1):157–164. doi: 10.1016/0016-5085(93)90021-4. [DOI] [PubMed] [Google Scholar]
- Willemer S., Elsässer H. P., Kern H. F., Adler G. Tubular complexes in cerulein- and oleic acid-induced pancreatitis in rats: glycoconjugate pattern, immunocytochemical, and ultrastructural findings. Pancreas. 1987;2(6):669–675. doi: 10.1097/00006676-198711000-00008. [DOI] [PubMed] [Google Scholar]
- Winsten S., Cehelyk B. A rapid micro diazo technique for measuring total bilirubin. Clin Chim Acta. 1969 Sep;25(3):441–446. doi: 10.1016/0009-8981(69)90206-x. [DOI] [PubMed] [Google Scholar]




