Abstract
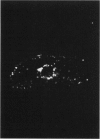
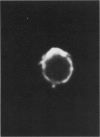
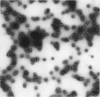
This paper examines the presence and characteristics of endocytosis by oesophageal epithelial cells. Biopsy specimens from normal and inflamed oesophagus were incubated in organ culture with fluorescent microspheres (0.1 and 0.01 microns diameter). These markers were taken into early endosomes and the lysosomes of both the smaller differentiating prickle cells and the larger mature squamous cells. Confocal and electron microscopy showed that markers passed to the early endosomes and the lysosomes by endocytosis. The process was energy dependent. Larger, 1 micron microspheres adhered to the epithelial cells but were not phagocytosed. Disaggregated cells were analysed by flow cytometry. Microspheres were endocytosed in proportion to the concentration in the culture medium in a dose dependent manner. Cells from inflamed oesophagus were significantly smaller (p = 0.013) and took up significantly more microspheres than cells from normal biopsy specimens (p = 0.015). In conclusion, endocytosis occurs in oesophageal epithelial cells and is increased in inflammation.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Browning T. H., Trier J. S. Organ culture of mucosal biopsies of human small intestine. J Clin Invest. 1969 Aug;48(8):1423–1432. doi: 10.1172/JCI106108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobson H., Pignatelli M., Hopwood D., D'Arrigo C. Cell adhesion molecules in oesophageal epithelium. Gut. 1994 Oct;35(10):1343–1347. doi: 10.1136/gut.35.10.1343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopwood D., Curtis M., Nicholson G., Milne G. The distribution and mobility of surface anionic groups of normal human oesophageal epithelium following interaction with cationized verritin. Virchows Arch B Cell Pathol Incl Mol Pathol. 1979;31(3):277–288. doi: 10.1007/BF02889944. [DOI] [PubMed] [Google Scholar]
- Hopwood D., Jankowski J., Milne G., Wormsley K. G. Flow cytometry of oesophageal mucosal biopsies; epidermal growth factor receptor, and CD15. J Pathol. 1992 Jul;167(3):321–326. doi: 10.1002/path.1711670310. [DOI] [PubMed] [Google Scholar]
- Hopwood D., Milne G., Jankowski J., Howat K., Johnston D., Wormsley K. G. Secretory and absorptive activity of oesophageal epithelium: evidence of circulating mucosubstances. Histochem J. 1994 Jan;26(1):41–49. [PubMed] [Google Scholar]
- Hopwood D., Milne G., Jankowski J., Howat K., Wormsley K. G. Uptake of horseradish peroxidase by human oesophageal explants over 24 h. Histochem J. 1991 Sep;23(9):409–414. doi: 10.1007/BF01042297. [DOI] [PubMed] [Google Scholar]
- Lundström A., Egelrud T. Cell shedding from human plantar skin in vitro: evidence of its dependence on endogenous proteolysis. J Invest Dermatol. 1988 Oct;91(4):340–343. doi: 10.1111/1523-1747.ep12475679. [DOI] [PubMed] [Google Scholar]
- Sanders D. S., Kerr M. A., Hopwood D., Coghill G., Milne G. A. Expression of the 3-fucosyl N-acetyllactosamine (CD 15) antigen in normal, metaplastic, dysplastic, and neoplastic squamous epithelia. J Pathol. 1988 Mar;154(3):255–262. doi: 10.1002/path.1711540308. [DOI] [PubMed] [Google Scholar]
- Tobey N. A., Orlando R. C. Mechanisms of acid injury to rabbit esophageal epithelium. Role of basolateral cell membrane acidification. Gastroenterology. 1991 Nov;101(5):1220–1228. doi: 10.1016/0016-5085(91)90070-2. [DOI] [PubMed] [Google Scholar]
- Tobey N. A., Reddy S. P., Keku T. O., Cragoe E. J., Jr, Orlando R. C. Studies of pHi in rabbit esophageal basal and squamous epithelial cells in culture. Gastroenterology. 1992 Sep;103(3):830–839. doi: 10.1016/0016-5085(92)90014-p. [DOI] [PubMed] [Google Scholar]
- Wolff K., Hönigsmann H. Permeability of the epidermis and the phagocytic activity of keratinocytes. Ultrastructural studies with thorotrast as a marker. J Ultrastruct Res. 1971 Jul;36(1):176–190. doi: 10.1016/s0022-5320(71)80096-5. [DOI] [PubMed] [Google Scholar]