Abstract
Transglutaminases are a family of Ca-dependent enzymes involved in various biological events. Circulating transglutaminase (factor XIIIa) is decreased in blood of patients with inflammatory bowel diseases. There is evidence that factor XIIIa and tissue type transglutaminase, present in cell cytosol, bind to various proteins of the extracellular matrix. This study examined the value of serum transglutaminase assay in the treatment and follow up of Crohn's disease and then investigated the intestinal location of both forms of transglutaminases by immunohistochemistry in normal and abnormal tissues. Serum transglutaminase activity was assayed in 36 patients with active Crohn's disease (CDAI > 150). Eighteen patients were studied prospectively from relapse into remission. A significant inverse correlation (p < 0.001) was found between circulating transglutaminase and Crohn's disease activity index; a correlation was also found between serum transglutaminase and serum orosomucoid (p < 0.01) and C reactive protein (p < 0.01). Patients were prospectively studied until clinical remission showed improvement in both their CDAI score mean (SD) (230 (46) to 72 (34), p < 0.01) and transglutaminase activity mean (SD) (0.61 (0.12) to 0.93 (0.13) mU/ml, p < 0.01). The immunohistochemistry assessment showed a colocalisation of factor XIIIa and tissue transglutaminase to the extracellular matrix of damaged tissues. In conclusion, these data confirm the value of serum transglutaminase assay as marker of Crohn's disease activity, extend the utility of serum transglutaminase assay to follow up of the disease, and emphasised the role of different types of transglutaminases in extracellular matrix assembly in the damaged tissues.
Full text
PDF
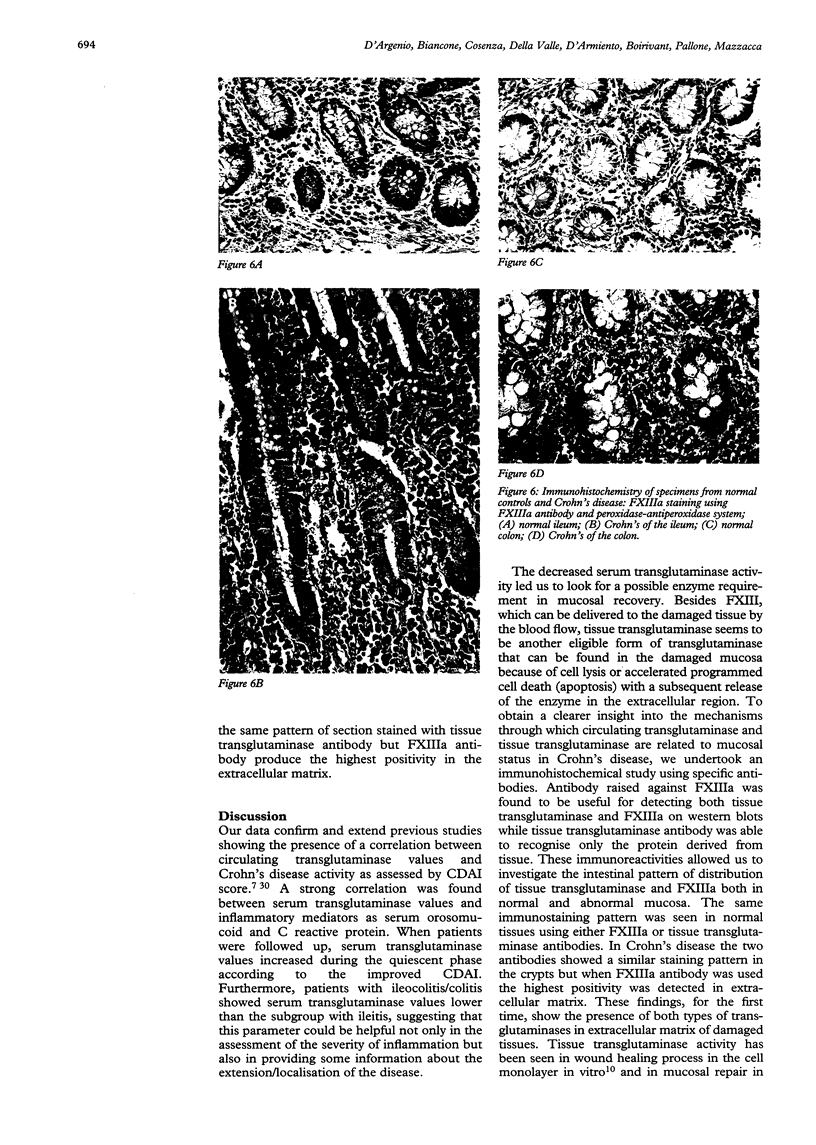

Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Achyuthan K. E., Mary A., Greenberg C. S. The binding sites on fibrin(ogen) for guinea pig liver transglutaminase are similar to those of blood coagulation factor XIII. Characterization of the binding of liver transglutaminase to fibrin. J Biol Chem. 1988 Oct 5;263(28):14296–14301. [PubMed] [Google Scholar]
- Ainbender E., Cabatu E. E., Guzman D. M., Sweet A. Y. Serum C-reactive protein and problems of newborn infants. J Pediatr. 1982 Sep;101(3):438–440. doi: 10.1016/s0022-3476(82)80080-2. [DOI] [PubMed] [Google Scholar]
- Allan A., Wyke J., Allan R. N., Morel P., Robinson M., Scott D. L., Alexander-Williams J. Plasma fibronectin in Crohn's disease. Gut. 1989 May;30(5):627–633. doi: 10.1136/gut.30.5.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry E. L., Mosher D. F. Factor XIII cross-linking of fibronectin at cellular matrix assembly sites. J Biol Chem. 1988 Jul 25;263(21):10464–10469. [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Bienvenu J., Sann L., Bienvenu F., Lahet C., Divry P., Cotte J., Bethenod M. Laser nephelometry of orosomucoid in serum of newborns: reference intervals and relation to bacterial infections. Clin Chem. 1981 May;27(5):721–726. [PubMed] [Google Scholar]
- Bruce S. E., Bjarnason I., Peters T. J. Human jejunal transglutaminase: demonstration of activity, enzyme kinetics and substrate specificity with special relation to gliadin and coeliac disease. Clin Sci (Lond) 1985 May;68(5):573–579. doi: 10.1042/cs0680573. [DOI] [PubMed] [Google Scholar]
- D'Argenio G., Cicacci C., Sorrentini I., Iovino P., Gatto A., Cosenza V., Mazzacca G. Serum transglutaminase in inflammatory bowel diseases. J Clin Gastroenterol. 1990 Aug;12(4):400–404. doi: 10.1097/00004836-199008000-00009. [DOI] [PubMed] [Google Scholar]
- D'Argenio G., Cosenza V., Sorrentini I., De Ritis F., Gatto A., Delle Cave M., D'Armiento F. P., Mazzacca G. Butyrate, mesalamine, and factor XIII in experimental colitis in the rat: effects on transglutaminase activity. Gastroenterology. 1994 Feb;106(2):399–404. doi: 10.1016/0016-5085(94)90598-3. [DOI] [PubMed] [Google Scholar]
- D'Argenio G., Sorrentini I., Ciacci C., Mazzacca G. Low serum transglutaminase in patients with intestinal lymphoma and alpha-chain disease. Am J Gastroenterol. 1990 Dec;85(12):1654–1655. [PubMed] [Google Scholar]
- D'Argenio G., Sorrentini I., Ciacci C., Spagnuolo S., Ventriglia R., de Chiara A., Mazzacca G. Human serum transglutaminase and coeliac disease: correlation between serum and mucosal activity in an experimental model of rat small bowel enteropathy. Gut. 1989 Jul;30(7):950–954. doi: 10.1136/gut.30.7.950. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Argenio G., Sorrentini I., Cosenza V., Gatto A., Iovino P., D'Armiento E. P., Baldassarre F., Mazzacca G. Serum and tissue transglutaminase correlates with the severity of inflammation in induced colitis in the rat. Scand J Gastroenterol. 1992;27(2):111–114. doi: 10.3109/00365529209165428. [DOI] [PubMed] [Google Scholar]
- Doumas B. T., Watson W. A., Biggs H. G. Albumin standards and the measurement of serum albumin with bromcresol green. Clin Chim Acta. 1971 Jan;31(1):87–96. doi: 10.1016/0009-8981(71)90365-2. [DOI] [PubMed] [Google Scholar]
- Fesus L., Thomazy V., Falus A. Induction and activation of tissue transglutaminase during programmed cell death. FEBS Lett. 1987 Nov 16;224(1):104–108. doi: 10.1016/0014-5793(87)80430-1. [DOI] [PubMed] [Google Scholar]
- Floyd E. E., Jetten A. M. Regulation of type I (epidermal) transglutaminase mRNA levels during squamous differentiation: down regulation by retinoids. Mol Cell Biol. 1989 Nov;9(11):4846–4851. doi: 10.1128/mcb.9.11.4846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Folk J. E., Finlayson J. S. The epsilon-(gamma-glutamyl)lysine crosslink and the catalytic role of transglutaminases. Adv Protein Chem. 1977;31:1–133. doi: 10.1016/s0065-3233(08)60217-x. [DOI] [PubMed] [Google Scholar]
- Folk J. E. Transglutaminases. Annu Rev Biochem. 1980;49:517–531. doi: 10.1146/annurev.bi.49.070180.002505. [DOI] [PubMed] [Google Scholar]
- Harvey R. F., Bradshaw J. M. A simple index of Crohn's-disease activity. Lancet. 1980 Mar 8;1(8167):514–514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- Hudson M., Wakefield A. J., Hutton R. A., Sankey E. A., Dhillon A. P., More L., Sim R., Pounder R. E. Factor XIIIA subunit and Crohn's disease. Gut. 1993 Jan;34(1):75–79. doi: 10.1136/gut.34.1.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ichinose A., Bottenus R. E., Davie E. W. Structure of transglutaminases. J Biol Chem. 1990 Aug 15;265(23):13411–13414. [PubMed] [Google Scholar]
- Ikura K., Nasu T., Yokota H., Tsuchiya Y., Sasaki R., Chiba H. Amino acid sequence of guinea pig liver transglutaminase from its cDNA sequence. Biochemistry. 1988 Apr 19;27(8):2898–2905. doi: 10.1021/bi00408a035. [DOI] [PubMed] [Google Scholar]
- Juprelle-Soret M., Wattiaux-De Coninck S., Wattiaux R. Subcellular localization of transglutaminase. Effect of collagen. Biochem J. 1988 Mar 1;250(2):421–427. doi: 10.1042/bj2500421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lorand L., Campbell-Wilkes L. K., Cooperstein L. A filter paper assay for transamidating enzymes using radioactive amine substrates. Anal Biochem. 1972 Dec;50(2):623–631. doi: 10.1016/0003-2697(72)90074-7. [DOI] [PubMed] [Google Scholar]
- Lorand L., Dailey J. E., Turner P. M. Fibronectin as a carrier for the transglutaminase from human erythrocytes. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1057–1059. doi: 10.1073/pnas.85.4.1057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modigliani R., Mary J. Y., Simon J. F., Cortot A., Soule J. C., Gendre J. P., Rene E. Clinical, biological, and endoscopic picture of attacks of Crohn's disease. Evolution on prednisolone. Groupe d'Etude Thérapeutique des Affections Inflammatoires Digestives. Gastroenterology. 1990 Apr;98(4):811–818. doi: 10.1016/0016-5085(90)90002-i. [DOI] [PubMed] [Google Scholar]
- Murtaugh M. P., Mehta K., Johnson J., Myers M., Juliano R. L., Davies P. J. Induction of tissue transglutaminase in mouse peritoneal macrophages. J Biol Chem. 1983 Sep 25;258(18):11074–11081. [PubMed] [Google Scholar]
- Reese A. C., Doran J. E., Callaway B. D., Mansberger A. R. Sequestration of fibronectin at the site of an injury. Adv Shock Res. 1982;8:119–127. [PubMed] [Google Scholar]
- Robbins A. B., Doran J. E., Reese A. C., Mansberger A. R., Jr Cold insoluble globulin levels in operative trauma: serum depletion, wound sequestration, and biological activity: an experimental and clinical study. Am Surg. 1980 Dec;46(12):663–672. [PubMed] [Google Scholar]
- Stadnicki A., Kloczko J., Nowak A., Sierka E., Sliwiński Z. Factor XIII subunits in relation to some other hemostatic parameters in ulcerative colitis. Am J Gastroenterol. 1991 Jun;86(6):690–693. [PubMed] [Google Scholar]
- Sternberger L. A., Hardy P. H., Jr, Cuculis J. J., Meyer H. G. The unlabeled antibody enzyme method of immunohistochemistry: preparation and properties of soluble antigen-antibody complex (horseradish peroxidase-antihorseradish peroxidase) and its use in identification of spirochetes. J Histochem Cytochem. 1970 May;18(5):315–333. doi: 10.1177/18.5.315. [DOI] [PubMed] [Google Scholar]
- Upchurch H. F., Conway E., Patterson M. K., Jr, Birckbichler P. J., Maxwell M. D. Cellular transglutaminase has affinity for extracellular matrix. In Vitro Cell Dev Biol. 1987 Nov;23(11):795–800. doi: 10.1007/BF02623682. [DOI] [PubMed] [Google Scholar]
- Upchurch H. F., Conway E., Patterson M. K., Jr, Maxwell M. D. Localization of cellular transglutaminase on the extracellular matrix after wounding: characteristics of the matrix bound enzyme. J Cell Physiol. 1991 Dec;149(3):375–382. doi: 10.1002/jcp.1041490304. [DOI] [PubMed] [Google Scholar]