Abstract
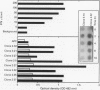
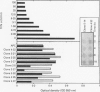
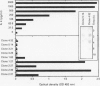
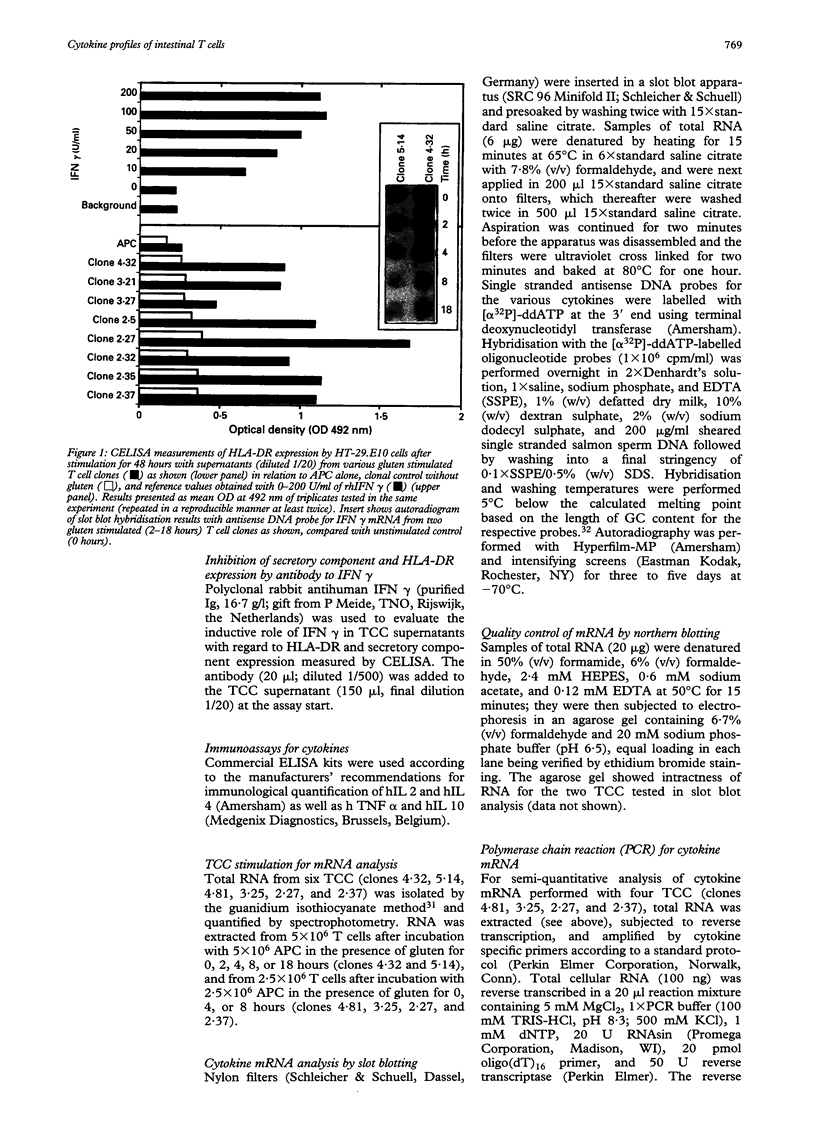
Coeliac disease is precipitated in susceptible subjects by ingestion of wheat gluten or gluten related prolamins from some other cereals. The disease is strongly associated with certain HLA-DQ heterodimers, for example, DQ2 (DQ alpha 1*0501, beta 1*0201) in most patients and apparently DQ8 (DQ alpha 1*0301, beta 1*0302) in a small subset. Gluten specific T cell clones (TCC) from coeliac intestinal lesions were recently established and found to be mainly restricted by HLA-DQ2 or HLA-DQ8. Antigen induced production of cytokines was studied in 15 TCC from three patients, 10 being DQ2 and five DQ8 restricted. Cell culture supernatants were prepared by stimulation with gluten peptides in the presence of DQ2+ or DQ8+ Epstein-Barr virus transformed B cells as antigen presenting cells (APC). Supernatants were analysed for cytokines by bioassays, ELISA, and CELISA. Cellular cytokine mRNA was analysed semi-quantitatively by slot blotting and polymerase chain reaction (PCR). All TCC were found to secrete interferon (IFN) gamma, often at high concentrations (> 2000 U/ml); some secreted in addition interleukin (IL) 4, IL 5, IL 6, IL 10, tumour necrosis factor (TNF), and transforming growth factor (TGF) beta. The last TCC thus displayed a Th0-like cytokine pattern. However, other TCC produced IFN gamma and TNF but no IL 4, or IL 5, compatible with a Th1-like pattern. In conclusion, most DQ8 restricted TCC seemed to fit with a Th0 profile whereas the DQ2 restricted TCC secreted cytokines more compatible with a Th1 pattern. The TCC supernatants induced upregulation of HLA-DR and secretory component (poly-Ig receptor) in the colonic adenocarcinoma cell line HT-29.E10, most probably reflecting mainly the high IFN gamma concentrations. This cytokine, particularly in combination with TNF alpha, might be involved in several pathological features of the coeliac lesion. The characterised cytokine profiles thus support the notion that mucosal T cells activated in situ by gluten in a DQ restricted fashion play a central part in the pathogenesis of coeliac disease.
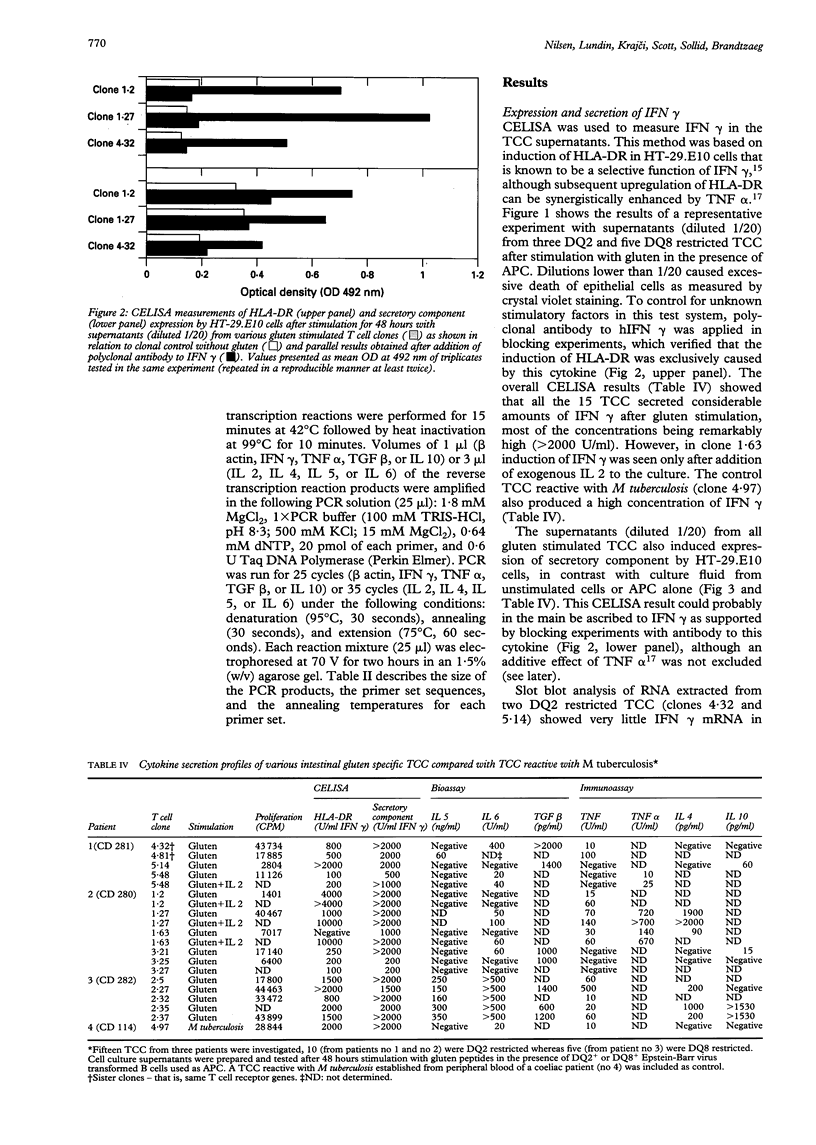
Full text
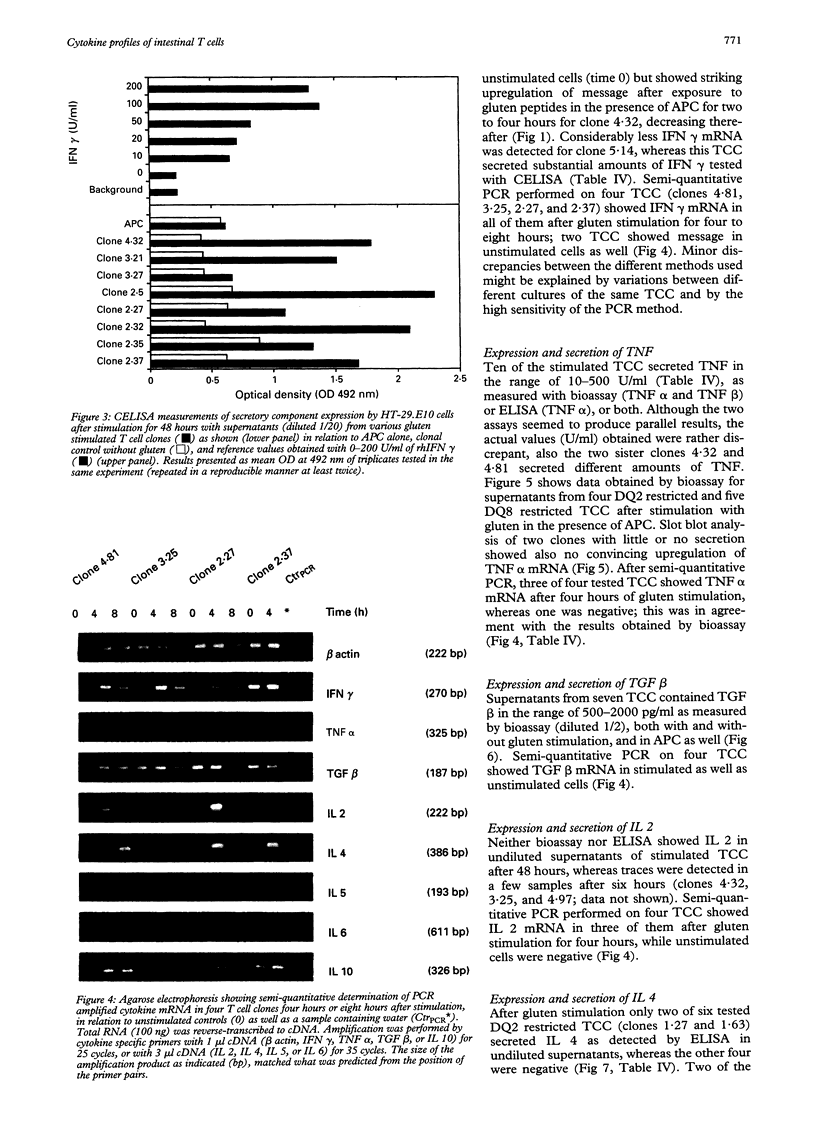
PDF

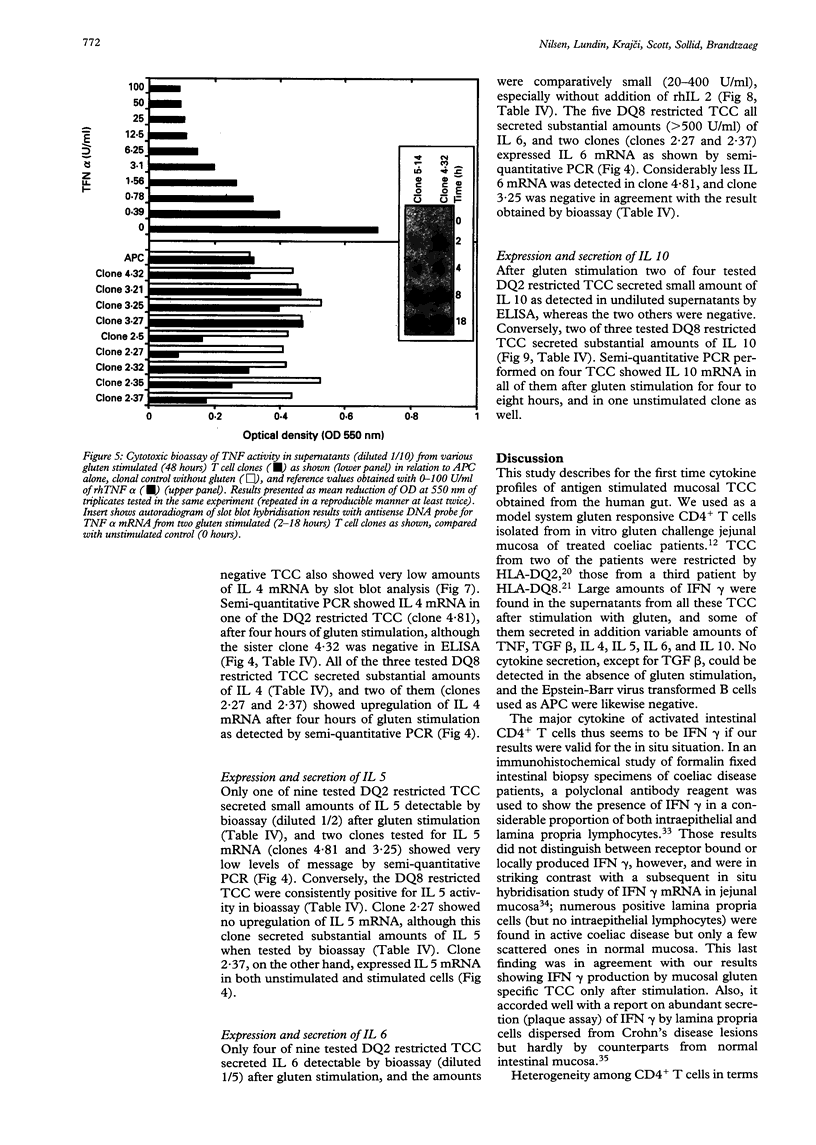
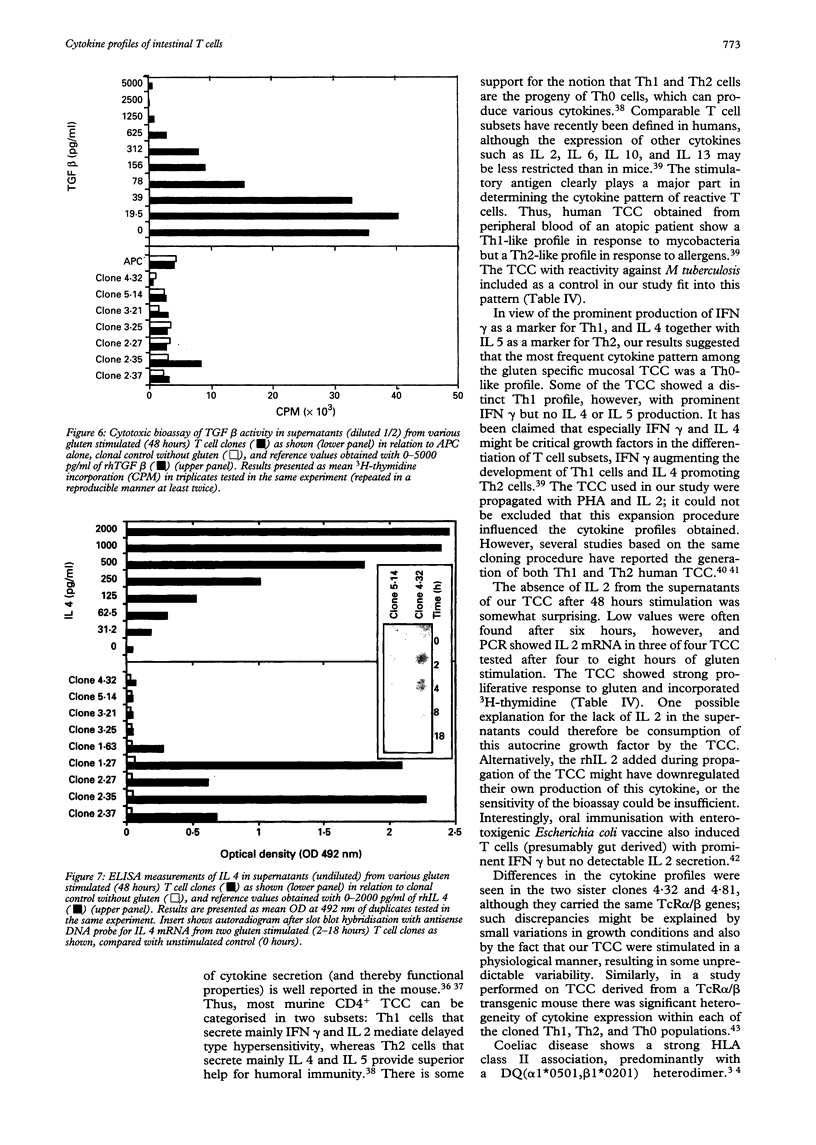
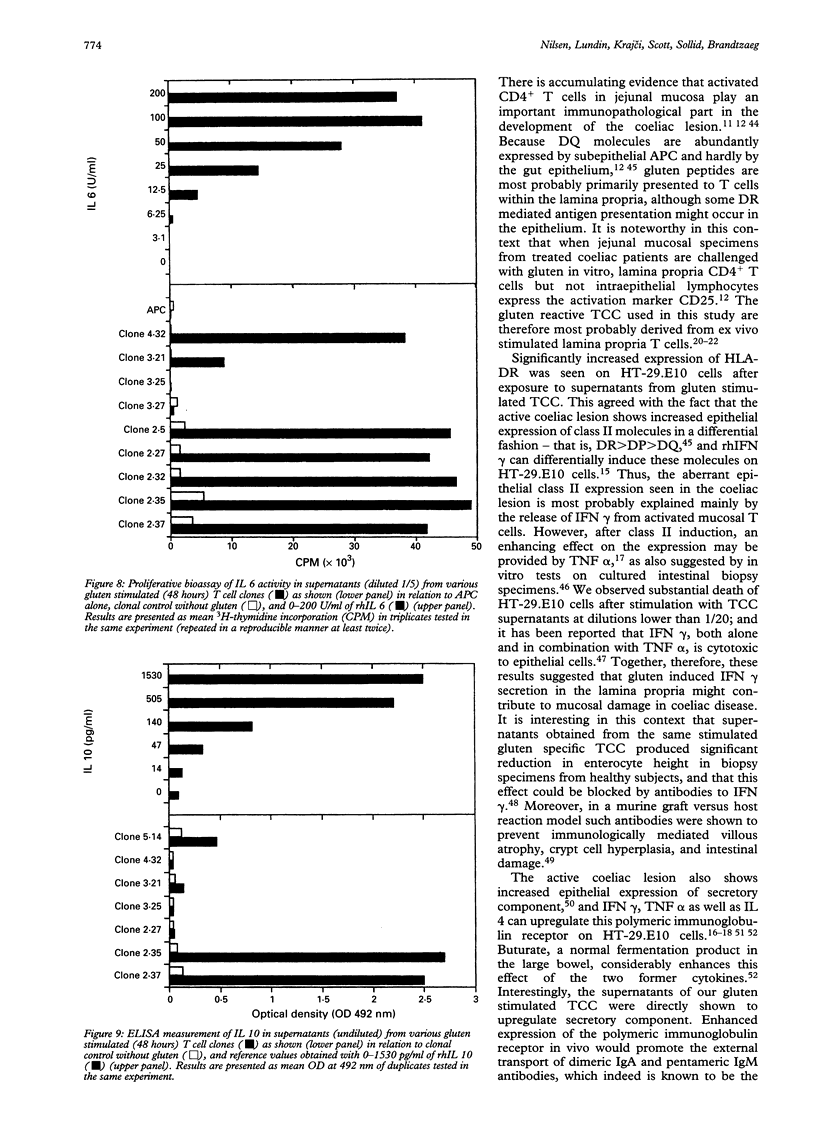


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bonner T. I., Brenner D. J., Neufeld B. R., Britten R. J. Reduction in the rate of DNA reassociation by sequence divergence. J Mol Biol. 1973 Dec 5;81(2):123–135. doi: 10.1016/0022-2836(73)90184-8. [DOI] [PubMed] [Google Scholar]
- Breese E., Braegger C. P., Corrigan C. J., Walker-Smith J. A., MacDonald T. T. Interleukin-2- and interferon-gamma-secreting T cells in normal and diseased human intestinal mucosa. Immunology. 1993 Jan;78(1):127–131. [PMC free article] [PubMed] [Google Scholar]
- Bucy R. P., Panoskaltsis-Mortari A., Huang G. Q., Li J., Karr L., Ross M., Russell J. H., Murphy K. M., Weaver C. T. Heterogeneity of single cell cytokine gene expression in clonal T cell populations. J Exp Med. 1994 Oct 1;180(4):1251–1262. doi: 10.1084/jem.180.4.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabtree J. E., Heatley R. V., Juby L. D., Howdle P. D., Losowsky M. S. Serum interleukin-2-receptor in coeliac disease: response to treatment and gluten challenge. Clin Exp Immunol. 1989 Sep;77(3):345–348. [PMC free article] [PubMed] [Google Scholar]
- Danielpour D., Dart L. L., Flanders K. C., Roberts A. B., Sporn M. B. Immunodetection and quantitation of the two forms of transforming growth factor-beta (TGF-beta 1 and TGF-beta 2) secreted by cells in culture. J Cell Physiol. 1989 Jan;138(1):79–86. doi: 10.1002/jcp.1041380112. [DOI] [PubMed] [Google Scholar]
- Deem R. L., Shanahan F., Targan S. R. Triggered human mucosal T cells release tumour necrosis factor-alpha and interferon-gamma which kill human colonic epithelial cells. Clin Exp Immunol. 1991 Jan;83(1):79–84. doi: 10.1111/j.1365-2249.1991.tb05592.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defrance T., Vanbervliet B., Brière F., Durand I., Rousset F., Banchereau J. Interleukin 10 and transforming growth factor beta cooperate to induce anti-CD40-activated naive human B cells to secrete immunoglobulin A. J Exp Med. 1992 Mar 1;175(3):671–682. doi: 10.1084/jem.175.3.671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Prete G. F., De Carli M., Ricci M., Romagnani S. Helper activity for immunoglobulin synthesis of T helper type 1 (Th1) and Th2 human T cell clones: the help of Th1 clones is limited by their cytolytic capacity. J Exp Med. 1991 Oct 1;174(4):809–813. doi: 10.1084/jem.174.4.809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espevik T., Nissen-Meyer J. A highly sensitive cell line, WEHI 164 clone 13, for measuring cytotoxic factor/tumor necrosis factor from human monocytes. J Immunol Methods. 1986 Dec 4;95(1):99–105. doi: 10.1016/0022-1759(86)90322-4. [DOI] [PubMed] [Google Scholar]
- Fais S., Maiuri L., Pallone F., De Vincenzi M., De Ritis G., Troncone R., Auricchio S. Gliadin induced changes in the expression of MHC-class II antigens by human small intestinal epithelium. Organ culture studies with coeliac disease mucosa. Gut. 1992 Apr;33(4):472–475. doi: 10.1136/gut.33.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson P., Rosella O., Nov R., Young G. Colonic epithelium is diffusely abnormal in ulcerative colitis and colorectal cancer. Gut. 1995 Jun;36(6):857–863. doi: 10.1136/gut.36.6.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halstensen T. S., Brandtzaeg P. Activated T lymphocytes in the celiac lesion: non-proliferative activation (CD25) of CD4+ alpha/beta cells in the lamina propria but proliferation (Ki-67) of alpha/beta and gamma/delta cells in the epithelium. Eur J Immunol. 1993 Feb;23(2):505–510. doi: 10.1002/eji.1830230231. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Hvatum M., Scott H., Fausa O., Brandtzaeg P. Association of subepithelial deposition of activated complement and immunoglobulin G and M response to gluten in celiac disease. Gastroenterology. 1992 Mar;102(3):751–759. doi: 10.1016/0016-5085(92)90155-r. [DOI] [PubMed] [Google Scholar]
- Halstensen T. S., Scott H., Fausa O., Brandtzaeg P. Gluten stimulation of coeliac mucosa in vitro induces activation (CD25) of lamina propria CD4+ T cells and macrophages but no crypt-cell hyperplasia. Scand J Immunol. 1993 Dec;38(6):581–590. doi: 10.1111/j.1365-3083.1993.tb03245.x. [DOI] [PubMed] [Google Scholar]
- Helle M., Boeije L., Aarden L. A. Functional discrimination between interleukin 6 and interleukin 1. Eur J Immunol. 1988 Oct;18(10):1535–1540. doi: 10.1002/eji.1830181010. [DOI] [PubMed] [Google Scholar]
- Karasuyama H., Melchers F. Establishment of mouse cell lines which constitutively secrete large quantities of interleukin 2, 3, 4 or 5, using modified cDNA expression vectors. Eur J Immunol. 1988 Jan;18(1):97–104. doi: 10.1002/eji.1830180115. [DOI] [PubMed] [Google Scholar]
- Kontakou M., Sturgess R. P., Przemioslo R. T., Limb G. A., Nelufer J. M., Ciclitira P. J. Detection of interferon gamma mRNA in the mucosa of patients with coeliac disease by in situ hybridisation. Gut. 1994 Aug;35(8):1037–1041. doi: 10.1136/gut.35.8.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krajci P., Taskén K., Kvale D., Brandtzaeg P. Interferon-gamma stimulation of messenger RNA for human secretory component (poly-Ig receptor) depends on continuous intermediate protein synthesis. Scand J Immunol. 1993 Feb;37(2):251–256. doi: 10.1111/j.1365-3083.1993.tb01763.x. [DOI] [PubMed] [Google Scholar]
- Kvale D., Brandtzaeg P. Constitutive and cytokine induced expression of HLA molecules, secretory component, and intercellular adhesion molecule-1 is modulated by butyrate in the colonic epithelial cell line HT-29. Gut. 1995 May;36(5):737–742. doi: 10.1136/gut.36.5.737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kvale D., Brandtzaeg P., Løvhaug D. Up-regulation of the expression of secretory component and HLA molecules in a human colonic cell line by tumour necrosis factor-alpha and gamma interferon. Scand J Immunol. 1988 Sep;28(3):351–357. doi: 10.1111/j.1365-3083.1988.tb01460.x. [DOI] [PubMed] [Google Scholar]
- Kvale D., Krajci P., Brandtzaeg P. Expression and regulation of adhesion molecules ICAM-1 (CD54) and LFA-3 (CD58) in human intestinal epithelial cell lines. Scand J Immunol. 1992 Jun;35(6):669–676. doi: 10.1111/j.1365-3083.1992.tb02973.x. [DOI] [PubMed] [Google Scholar]
- Lionetti P., Breese E., Braegger C. P., Murch S. H., Taylor J., MacDonald T. T. T-cell activation can induce either mucosal destruction or adaptation in cultured human fetal small intestine. Gastroenterology. 1993 Aug;105(2):373–381. doi: 10.1016/0016-5085(93)90710-t. [DOI] [PubMed] [Google Scholar]
- Lundin K. E., Gjertsen H. A., Scott H., Sollid L. M., Thorsby E. Function of DQ2 and DQ8 as HLA susceptibility molecules in celiac disease. Hum Immunol. 1994 Sep;41(1):24–27. doi: 10.1016/0198-8859(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Lundin K. E., Scott H., Fausa O., Thorsby E., Sollid L. M. T cells from the small intestinal mucosa of a DR4, DQ7/DR4, DQ8 celiac disease patient preferentially recognize gliadin when presented by DQ8. Hum Immunol. 1994 Dec;41(4):285–291. doi: 10.1016/0198-8859(94)90047-7. [DOI] [PubMed] [Google Scholar]
- Lundin K. E., Scott H., Hansen T., Paulsen G., Halstensen T. S., Fausa O., Thorsby E., Sollid L. M. Gliadin-specific, HLA-DQ(alpha 1*0501,beta 1*0201) restricted T cells isolated from the small intestinal mucosa of celiac disease patients. J Exp Med. 1993 Jul 1;178(1):187–196. doi: 10.1084/jem.178.1.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- MacDonald T. T., Spencer J. Evidence that activated mucosal T cells play a role in the pathogenesis of enteropathy in human small intestine. J Exp Med. 1988 Apr 1;167(4):1341–1349. doi: 10.1084/jem.167.4.1341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Stafford J. Interferon-gamma directly affects barrier function of cultured intestinal epithelial monolayers. J Clin Invest. 1989 Feb;83(2):724–727. doi: 10.1172/JCI113938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mantovani V., Corazza G. R., Bragliani M., Frisoni M., Zaniboni M. G., Gasbarrini G. Asp57-negative HLA DQ beta chain and DQA1*0501 allele are essential for the onset of DQw2-positive and DQw2-negative coeliac disease. Clin Exp Immunol. 1993 Jan;91(1):153–156. doi: 10.1111/j.1365-2249.1993.tb03371.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marsh M. N. Gluten, major histocompatibility complex, and the small intestine. A molecular and immunobiologic approach to the spectrum of gluten sensitivity ('celiac sprue'). Gastroenterology. 1992 Jan;102(1):330–354. [PubMed] [Google Scholar]
- McGhee J. R., Mestecky J., Elson C. O., Kiyono H. Regulation of IgA synthesis and immune response by T cells and interleukins. J Clin Immunol. 1989 May;9(3):175–199. doi: 10.1007/BF00916814. [DOI] [PubMed] [Google Scholar]
- Mosmann T. R., Cherwinski H., Bond M. W., Giedlin M. A., Coffman R. L. Two types of murine helper T cell clone. I. Definition according to profiles of lymphokine activities and secreted proteins. J Immunol. 1986 Apr 1;136(7):2348–2357. [PubMed] [Google Scholar]
- Mosmann T. R., Coffman R. L. TH1 and TH2 cells: different patterns of lymphokine secretion lead to different functional properties. Annu Rev Immunol. 1989;7:145–173. doi: 10.1146/annurev.iy.07.040189.001045. [DOI] [PubMed] [Google Scholar]
- Mosmann T. Rapid colorimetric assay for cellular growth and survival: application to proliferation and cytotoxicity assays. J Immunol Methods. 1983 Dec 16;65(1-2):55–63. doi: 10.1016/0022-1759(83)90303-4. [DOI] [PubMed] [Google Scholar]
- Mowat A. M. Antibodies to IFN-gamma prevent immunologically mediated intestinal damage in murine graft-versus-host reaction. Immunology. 1989 Sep;68(1):18–23. [PMC free article] [PubMed] [Google Scholar]
- Parronchi P., Macchia D., Piccinni M. P., Biswas P., Simonelli C., Maggi E., Ricci M., Ansari A. A., Romagnani S. Allergen- and bacterial antigen-specific T-cell clones established from atopic donors show a different profile of cytokine production. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4538–4542. doi: 10.1073/pnas.88.10.4538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips J. O., Everson M. P., Moldoveanu Z., Lue C., Mestecky J. Synergistic effect of IL-4 and IFN-gamma on the expression of polymeric Ig receptor (secretory component) and IgA binding by human epithelial cells. J Immunol. 1990 Sep 15;145(6):1740–1744. [PubMed] [Google Scholar]
- Romagnani S. Human TH1 and TH2 subsets: regulation of differentiation and role in protection and immunopathology. Int Arch Allergy Immunol. 1992;98(4):279–285. doi: 10.1159/000236199. [DOI] [PubMed] [Google Scholar]
- Scott H., Brandtzaeg P., Solheim B. G., Thorsby E. Relation between HLA-DR-like antigens and secretory component (SC) in jejunal epithelium of patients with coeliac disease or dermatitis herpetiformis. Clin Exp Immunol. 1981 May;44(2):233–238. [PMC free article] [PubMed] [Google Scholar]
- Scott H., Sollid L. M., Fausa O., Brandtzaeg P., Thorsby E. Expression of major histocompatibility complex class II subregion products by jejunal epithelium in patients with coeliac disease. Scand J Immunol. 1987 Nov;26(5):563–571. doi: 10.1111/j.1365-3083.1987.tb02290.x. [DOI] [PubMed] [Google Scholar]
- Shields J., Bernasconi L. M., Benotto W., Shaw A. R., Mazzei G. J. Production of a 26,000-dalton interleukin 1 inhibitor by human monocytes is regulated by granulocyte-macrophage colony-stimulating factor. Cytokine. 1990 Mar;2(2):122–128. doi: 10.1016/1043-4666(90)90006-f. [DOI] [PubMed] [Google Scholar]
- Sollid L. M., Gaudernack G., Markussen G., Kvale D., Brandtzaeg P., Thorsby E. Induction of various HLA class II molecules in a human colonic adenocarcinoma cell line. Scand J Immunol. 1987 Feb;25(2):175–180. doi: 10.1111/j.1365-3083.1987.tb01061.x. [DOI] [PubMed] [Google Scholar]
- Sollid L. M., Kvale D., Brandtzaeg P., Markussen G., Thorsby E. Interferon-gamma enhances expression of secretory component, the epithelial receptor for polymeric immunoglobulins. J Immunol. 1987 Jun 15;138(12):4303–4306. [PubMed] [Google Scholar]
- Sollid L. M., Markussen G., Ek J., Gjerde H., Vartdal F., Thorsby E. Evidence for a primary association of celiac disease to a particular HLA-DQ alpha/beta heterodimer. J Exp Med. 1989 Jan 1;169(1):345–350. doi: 10.1084/jem.169.1.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sollid L. M., Thorsby E. HLA susceptibility genes in celiac disease: genetic mapping and role in pathogenesis. Gastroenterology. 1993 Sep;105(3):910–922. doi: 10.1016/0016-5085(93)90912-v. [DOI] [PubMed] [Google Scholar]
- Spurkland A., Sollid L. M., Polanco I., Vartdal F., Thorsby E. HLA-DR and -DQ genotypes of celiac disease patients serologically typed to be non-DR3 or non-DR5/7. Hum Immunol. 1992 Nov;35(3):188–192. doi: 10.1016/0198-8859(92)90104-u. [DOI] [PubMed] [Google Scholar]
- Sturgess R. P., Hooper L. B., Spencer J., Hung C. H., Nelufer J. M., Ciclitira P. J. Effects of interferon-gamma and tumour necrosis factor-alpha on epithelial HLA class-II expression on jejunal mucosal biopsy specimens cultured in vitro. Scand J Gastroenterol. 1992 Nov;27(11):907–911. doi: 10.3109/00365529209000161. [DOI] [PubMed] [Google Scholar]
- Sturgess R. P., Macartney J. C., Makgoba M. W., Hung C. H., Haskard D. O., Ciclitira P. J. Differential upregulation of intercellular adhesion molecule-1 in coeliac disease. Clin Exp Immunol. 1990 Dec;82(3):489–492. doi: 10.1111/j.1365-2249.1990.tb05477.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swain S. L., Bradley L. M., Croft M., Tonkonogy S., Atkins G., Weinberg A. D., Duncan D. D., Hedrick S. M., Dutton R. W., Huston G. Helper T-cell subsets: phenotype, function and the role of lymphokines in regulating their development. Immunol Rev. 1991 Oct;123:115–144. doi: 10.1111/j.1600-065x.1991.tb00608.x. [DOI] [PubMed] [Google Scholar]
- Trier J. S. Celiac sprue. N Engl J Med. 1991 Dec 12;325(24):1709–1719. doi: 10.1056/NEJM199112123252406. [DOI] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennerås C., Svennerholm A. M., Czerkinsky C. Vaccine-specific T cells in human peripheral blood after oral immunization with an inactivated enterotoxigenic Escherichia coli vaccine. Infect Immun. 1994 Mar;62(3):874–879. doi: 10.1128/iai.62.3.874-879.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- al-Dawoud A., Nakshabendi I., Foulis A., Mowat A. M. Immunohistochemical analysis of mucosal gamma-interferon production in coeliac disease. Gut. 1992 Nov;33(11):1482–1486. doi: 10.1136/gut.33.11.1482. [DOI] [PMC free article] [PubMed] [Google Scholar]