Abstract
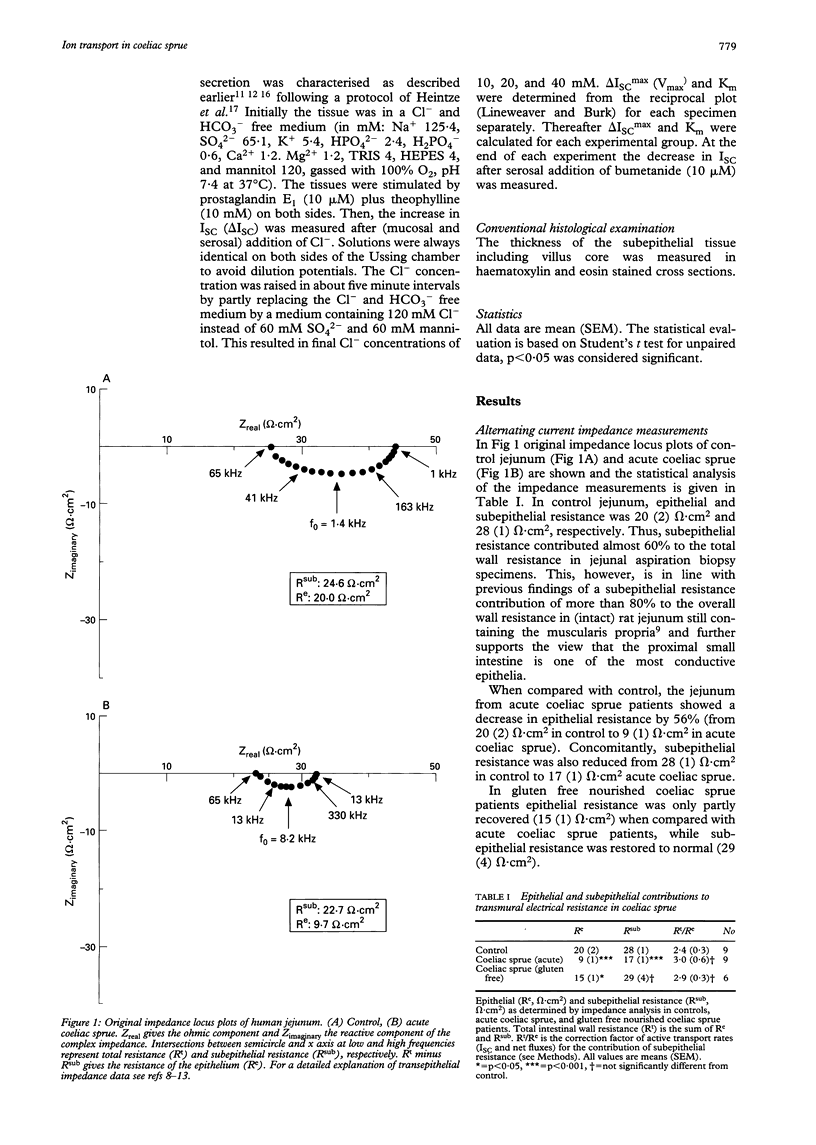
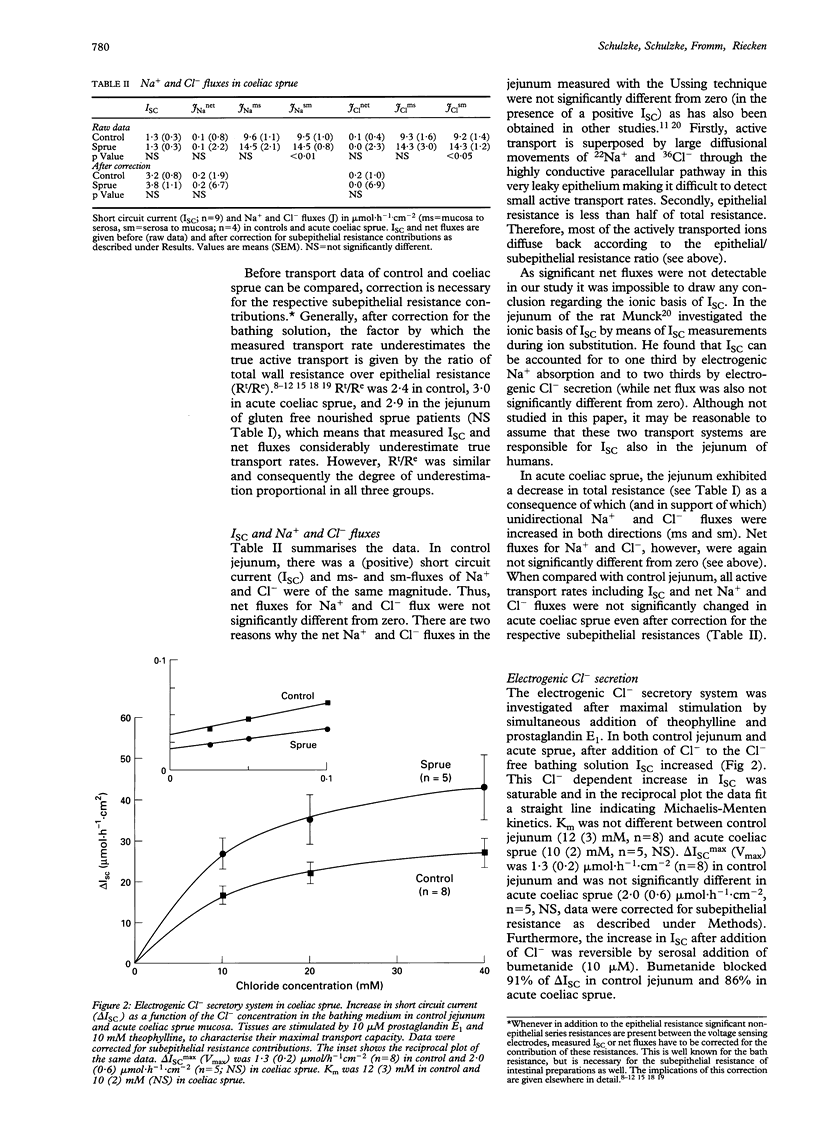
Epithelial barrier function and ion transport was studied in coeliac sprue using a miniaturised Ussing device for measurements on diagnostic aspiration biopsy specimens from the jejunum of untreated or gluten free nourished sprue patients, or from healthy controls. Pure epithelial resistance (Re) indicating epithelial barrier function was determined by transmural alternating current impedance analysis. It was reduced by 56% in acute sprue mean (SEM) (9 (1) omega.cm2) compared with controls (20(2) omega.cm2). In gluten free nourished sprue patients Re was only partly recovered (15 (1) omega.cm2). Subepithelial resistance (Rsub) was also changed from 28 (1) omega.cm2- in control to 17 (1) omega.cm2 in acute sprue because of the change in mucosal architecture, but was unchanged in gluten free nourished sprue patients (29 (4) omega.cm2). In acute sprue, unidirectional Na+ and Cl- fluxes were increased in both directions as a consequence of the decreased resistance. However, short circuit current (ISC) as well as Na+ and Cl- net fluxes were not significantly different from control. Subsequently, the electrogenic Cl- secretory system was investigated. After maximal stimulation with theophylline and prostaglandin E1, a Cl(-)-dependent increase in ISC was obtained in the sprue mucosa and control jejunum. It showed saturation characteristics and was blockable by serosal bumetanide. When compared with control, neither Km nor Vmax of this electrogenic Cl- secretion was significantly changed in coeliac sprue. In conclusion, a miniaturised Ussing device was used for transport measurements on intestinal biopsy specimens. In acute coeliac disease, the epithelial barrier of the jejunum was seriously disturbed. The active electrogenic Cl- secretory transport system was present in the sprue mucosa, but was not activated in the Ussing chamber in vitro when compared with control jejunum.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Archampong E. Q., Harris J., Clark C. G. The absorption and secretion of water and electrolytes across the healthy and the diseased human colonic mucosa measured in vitro. Gut. 1972 Nov;13(11):880–886. doi: 10.1136/gut.13.11.880. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloch R., Menge H., Lingelbach B., Lorenz-Meyer H., Haberich F. J., Riecken E. O. The relationship between structure and function of small intestine in patients with a sprue syndrome and in healthy controls. Klin Wochenschr. 1973 Dec 1;51(23):1151–1158. doi: 10.1007/BF01468564. [DOI] [PubMed] [Google Scholar]
- Cobden I., Dickinson R. J., Rothwell J., Axon A. T. Intestinal permeability assessed by excretion ratios of two molecules: results in coeliac disease. Br Med J. 1978 Oct 14;2(6144):1060–1060. doi: 10.1136/bmj.2.6144.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fordtran J. S., Rector F. C., Locklear T. W., Ewton M. F. Water and solute movement in the small intestine of patients with sprue. J Clin Invest. 1967 Mar;46(3):287–298. doi: 10.1172/JCI105531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fromm M., Palant C. E., Bentzel C. J., Hegel U. Protamine reversibly decreases paracellular cation permeability in Necturus gallbladder. J Membr Biol. 1985;87(2):141–150. doi: 10.1007/BF01870660. [DOI] [PubMed] [Google Scholar]
- Fromm M., Schulzke J. D., Hegel U. Epithelial and subepithelial contributions to transmural electrical resistance of intact rat jejunum, in vitro. Pflugers Arch. 1985 Dec;405(4):400–402. doi: 10.1007/BF00595695. [DOI] [PubMed] [Google Scholar]
- Hegel U., Fromm M. Electrical measurements in large intestine (including caecum, colon, rectum). Methods Enzymol. 1990;192:459–484. doi: 10.1016/0076-6879(90)92087-t. [DOI] [PubMed] [Google Scholar]
- Heintze K., Stewart C. P., Frizzell R. A. Sodium-dependent chloride secretion across rabbit descending colon. Am J Physiol. 1983 Apr;244(4):G357–G365. doi: 10.1152/ajpgi.1983.244.4.G357. [DOI] [PubMed] [Google Scholar]
- Jodal M., Hallbäck D. A., Lundgren O. Tissue osmolality in intestinal villi during luminal perfusion with isotonic electrolyte solutions. Acta Physiol Scand. 1978 Jan;102(1):94–107. doi: 10.1111/j.1748-1716.1978.tb06049.x. [DOI] [PubMed] [Google Scholar]
- MCCARTHY C. F., GOUGH K. R., RODRIGUEZ M., READ A. E. PERORAL INTESTINAL MUCOSAL BIOPSY WITH A SMALL CROSBY CAPSULE. Br Med J. 1964 Jun 20;1(5398):1620–1620. doi: 10.1136/bmj.1.5398.1620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madara J. L., Trier J. S. Structural abnormalities of jejunal epithelial cell membranes in celiac sprue. Lab Invest. 1980 Sep;43(3):254–261. [PubMed] [Google Scholar]
- Menzies I. S., Laker M. F., Pounder R., Bull J., Heyer S., Wheeler P. G., Creamer B. Abnormal intestinal permeability to sugars in villous atrophy. Lancet. 1979 Nov 24;2(8152):1107–1109. doi: 10.1016/s0140-6736(79)92507-8. [DOI] [PubMed] [Google Scholar]
- Munck B. G. Effects of sugar and amino acid transport on transepithelial fluxes of sodium and chloride of short circuited rat jejunum. J Physiol. 1972 Jun;223(3):699–717. doi: 10.1113/jphysiol.1972.sp009870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palade G. E., Simionescu M., Simionescu N. Structural aspects of the permeability of the microvascular endothelium. Acta Physiol Scand Suppl. 1979;463:11–32. [PubMed] [Google Scholar]
- SCHULTZ S. G., ZALUSKY R. ION TRANSPORT IN ISOLATED RABBIT ILEUM. I. SHORT-CIRCUIT CURRENT AND NA FLUXES. J Gen Physiol. 1964 Jan;47:567–584. doi: 10.1085/jgp.47.3.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schifferdecker E., Frömter E. The AC impedance of Necturus gallbladder epithelium. Pflugers Arch. 1978 Nov 14;377(2):125–133. doi: 10.1007/BF00582842. [DOI] [PubMed] [Google Scholar]
- Schmid W. C., Phillips S. F., Summerskill W. H. Jejunal secretion of electrolytes and water in nontropical sprue. J Lab Clin Med. 1969 May;73(5):772–783. [PubMed] [Google Scholar]
- Schulzke J. D., Fromm M., Bentzel C. J., Zeitz M., Menge H., Riecken E. O. Ion transport in the experimental short bowel syndrome of the rat. Gastroenterology. 1992 Feb;102(2):497–504. doi: 10.1016/0016-5085(92)90096-h. [DOI] [PubMed] [Google Scholar]
- Schulzke J. D., Fromm M., Hegel U., Riecken E. O. Ion transport and enteric nervous system (ENS) in rat rectal colon: mechanical stretch causes electrogenic Cl-secretion via plexus Meissner and amiloride-sensitive electrogenic Na-absorption is not affected by intramural neurons. Pflugers Arch. 1989 Jun;414(2):216–221. doi: 10.1007/BF00580966. [DOI] [PubMed] [Google Scholar]
- Schulzke J. D., Fromm M., Menge H., Riecken E. O. Impaired intestinal sodium and chloride transport in the blind loop syndrome of the rat. Gastroenterology. 1987 Mar;92(3):693–698. doi: 10.1016/0016-5085(87)90019-9. [DOI] [PubMed] [Google Scholar]
- Sjöqvist A., Beeuwkes R., 3rd Villus and crypt electrolyte and fluid transport during intestinal secretion. Acta Physiol Scand. 1990 May;139(1):203–210. doi: 10.1111/j.1748-1716.1990.tb08913.x. [DOI] [PubMed] [Google Scholar]
- Tai Y. H., Tai C. Y. The conventional short-circuiting technique under-short-circuits most epithelia. J Membr Biol. 1981 Apr 30;59(3):173–177. doi: 10.1007/BF01875423. [DOI] [PubMed] [Google Scholar]
- USSING H. H., ZERAHN K. Active transport of sodium as the source of electric current in the short-circuited isolated frog skin. Acta Physiol Scand. 1951 Aug 25;23(2-3):110–127. doi: 10.1111/j.1748-1716.1951.tb00800.x. [DOI] [PubMed] [Google Scholar]
- Ukabam S. O., Cooper B. T. Small intestinal permeability to mannitol, lactulose, and polyethylene glycol 400 in celiac disease. Dig Dis Sci. 1984 Sep;29(9):809–816. doi: 10.1007/BF01318423. [DOI] [PubMed] [Google Scholar]


