Abstract
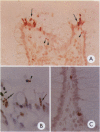
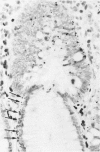
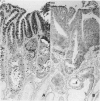
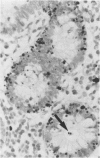
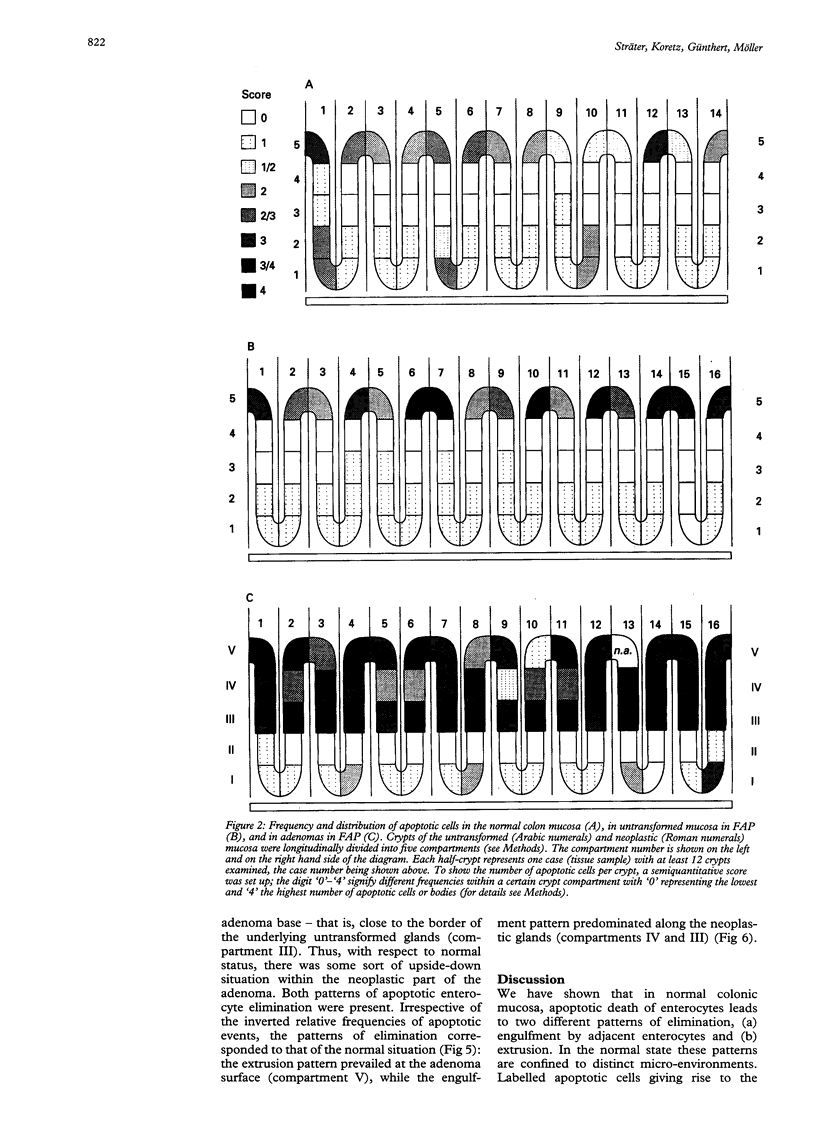
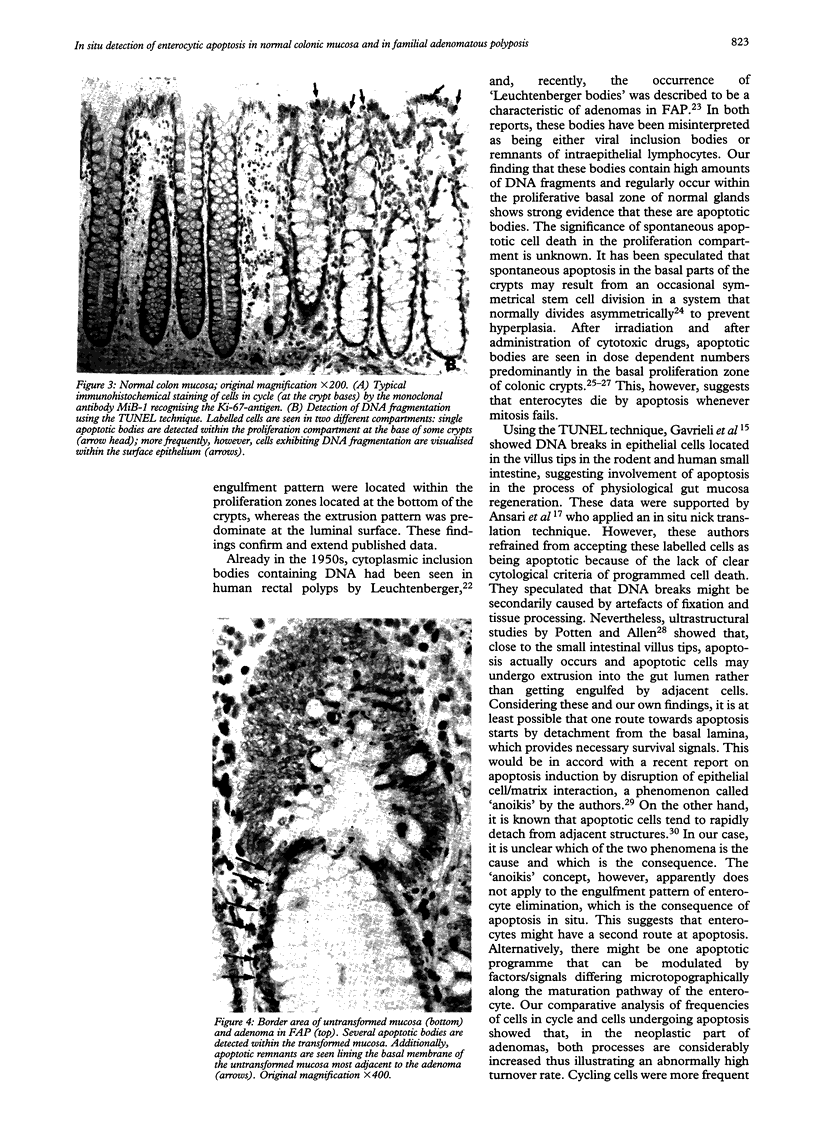
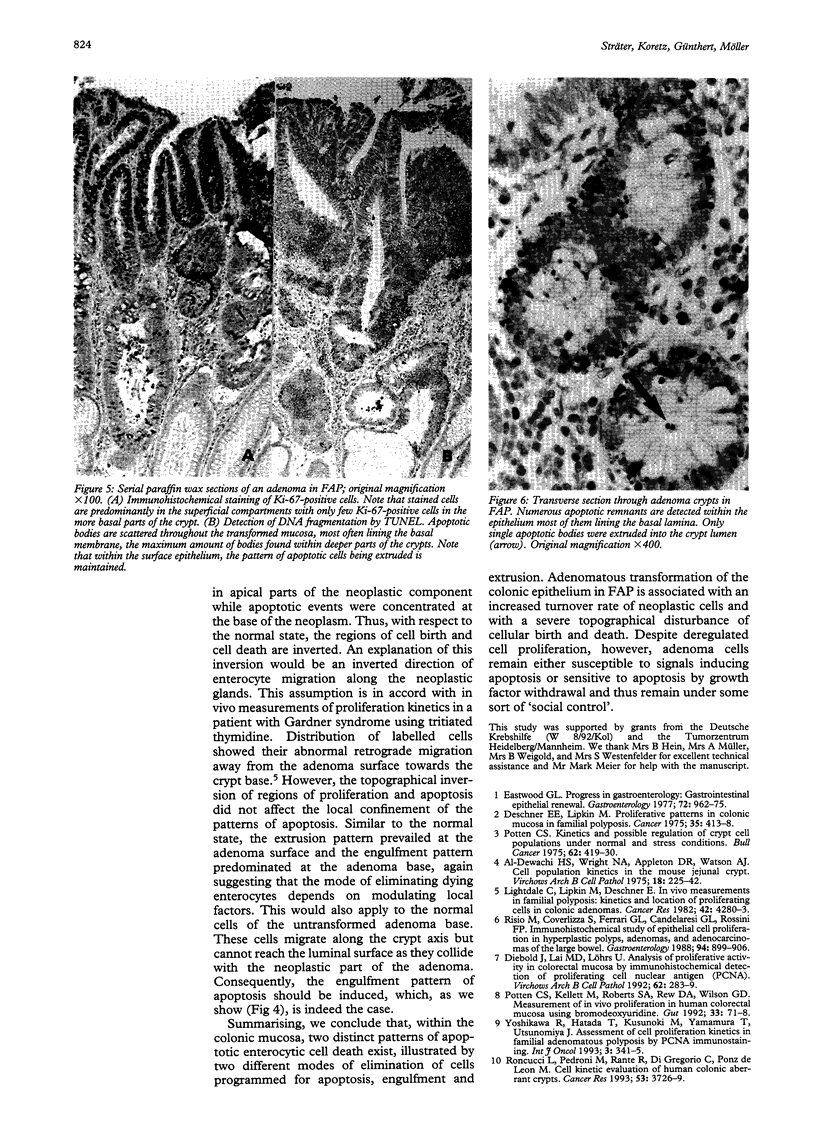
Physiological regeneration of colonic epithelium entails proliferation at the crypt base and cell loss by shedding or cell death. The aim of this study was to localise and assess the rate of apoptosis in normal and neoplastic colonic epithelium with respect to zones of proliferation. Familial adenomatous polyposis (FAP) was chosen as a model to study neoplastic transformation of colonic mucosa at an early stage. Apoptotic cells were detected in situ by TdT-mediated biotin-dUTP nick end labelling (TUNEL) in parallel to cells in cycle determined by Ki-67 immunohistochemistry using the monoclonal antibody MiB-1. By detection of genomic fragmentation, two different patterns of enterocytic apoptosis emerged: (a) apoptotic bodies being engulfed by adjacent epithelial cells, and (b) apoptotic cells with only subtle morphological changes being extruded into the gut lumen. The engulfment pattern was seen predominantly in the crypts of the normal colonic mucosa and, although very rare, was clearly confined to the basal proliferation compartment. The extrusion pattern was restricted to the luminal mucosal surface. Adenomas of FAP showed highly increased numbers of apoptotic bodies, which were scattered throughout the transformed mucosa. Both patterns of apoptosis were topographically intermingled although the extrusion pattern prevailed at the luminal adenoma surfaces. Whereas cells in cycle were somewhat more numerous in the upper parts of the crypts, apoptosis occurred with increased frequency at sites beneath the proliferation maximum suggesting an inverted direction of epithelial cell migration in adenomas. These results suggest two distinct routes towards enterocytic apoptosis in the colonic mucosa leading to engulfment or extrusion of dying cells. Adenomatous transformation of colon epithelium is associated with a considerable increase of the cellular turnover rate and with a severe disturbance of the microtopographical localisation of birth and death of enterocytes.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Al-Dewachi H. S., Wright N. A., Appleton D. R., Watson A. J. Cell population kinetics in the mouse jejunal crypt. Virchows Arch B Cell Pathol. 1975 Jul 18;18(3):225–242. doi: 10.1007/BF02889250. [DOI] [PubMed] [Google Scholar]
- Ansari B., Coates P. J., Greenstein B. D., Hall P. A. In situ end-labelling detects DNA strand breaks in apoptosis and other physiological and pathological states. J Pathol. 1993 May;170(1):1–8. doi: 10.1002/path.1711700102. [DOI] [PubMed] [Google Scholar]
- COLE J. W., McKALEN A. Observations of cell renewal in human rectal mucosa in vivo with thymidine-H3. Gastroenterology. 1961 Aug;41:122–125. [PubMed] [Google Scholar]
- Deschner E. E., Lipkin M. Proliferative patterns in colonic mucosa in familial polyposis. Cancer. 1975 Feb;35(2):413–418. doi: 10.1002/1097-0142(197502)35:2<413::aid-cncr2820350217>3.0.co;2-b. [DOI] [PubMed] [Google Scholar]
- Deschner E. E., Raicht R. F. Kinetic and morphologic alterations in the colon of a patient with multiple polyposis. Cancer. 1981 May 15;47(10):2440–2445. doi: 10.1002/1097-0142(19810515)47:10<2440::aid-cncr2820471022>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- Diebold J., Lai M. D., Löhrs U. Analysis of proliferative activity in colorectal mucosa by immunohistochemical detection of proliferating cell nuclear antigen (PCNA). Methodological aspects and application to routine diagnostic material. Virchows Arch B Cell Pathol Incl Mol Pathol. 1992;62(5):283–289. doi: 10.1007/BF02899694. [DOI] [PubMed] [Google Scholar]
- Eastwood G. L. Gastrointestinal epithelial renewal. Gastroenterology. 1977 May;72(5 Pt 1):962–975. [PubMed] [Google Scholar]
- Frisch S. M., Francis H. Disruption of epithelial cell-matrix interactions induces apoptosis. J Cell Biol. 1994 Feb;124(4):619–626. doi: 10.1083/jcb.124.4.619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gavrieli Y., Sherman Y., Ben-Sasson S. A. Identification of programmed cell death in situ via specific labeling of nuclear DNA fragmentation. J Cell Biol. 1992 Nov;119(3):493–501. doi: 10.1083/jcb.119.3.493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ijiri K., Potten C. S. Further studies on the response of intestinal crypt cells of different hierarchical status to eighteen different cytotoxic agents. Br J Cancer. 1987 Feb;55(2):113–123. doi: 10.1038/bjc.1987.25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Key G., Becker M. H., Baron B., Duchrow M., Schlüter C., Flad H. D., Gerdes J. New Ki-67-equivalent murine monoclonal antibodies (MIB 1-3) generated against bacterially expressed parts of the Ki-67 cDNA containing three 62 base pair repetitive elements encoding for the Ki-67 epitope. Lab Invest. 1993 Jun;68(6):629–636. [PubMed] [Google Scholar]
- LEUCHTENBERGER C. Cytoplasmic "inclusion bodies" containing desoxyribose nucleic acid (DNA) in cells of human rectal polyps. Lab Invest. 1954 Mar-Apr;3(2):132–142. [PubMed] [Google Scholar]
- LIPKIN M., SHERLOCK P., BELL B. CELL PROLIFERATION KINETICS IN THE GASTROINTESTINAL TRACT OF MAN. II. CELL RENEWAL IN STOMACH, ILEUM, COLON, AND RECTUM. Gastroenterology. 1963 Dec;45:721–729. [PubMed] [Google Scholar]
- Lightdale C., Lipkin M., Deschner E. In vivo measurements in familial polyposis: kinetics and location of proliferating cells in colonic adenomas. Cancer Res. 1982 Oct;42(10):4280–4283. [PubMed] [Google Scholar]
- Lipkin M. Biomarkers of increased susceptibility to gastrointestinal cancer: new application to studies of cancer prevention in human subjects. Cancer Res. 1988 Jan 15;48(2):235–245. [PubMed] [Google Scholar]
- Loeffler M., Birke A., Winton D., Potten C. Somatic mutation, monoclonality and stochastic models of stem cell organization in the intestinal crypt. J Theor Biol. 1993 Feb 21;160(4):471–491. doi: 10.1006/jtbi.1993.1031. [DOI] [PubMed] [Google Scholar]
- MACDONALD W. C., TRIER J. S., EVERETT N. B. CELL PROLIFERATION AND MIGRATION IN THE STOMACH, DUODENUM, AND RECTUM OF MAN: RADIOAUTOGRAPHIC STUDIES. Gastroenterology. 1964 Apr;46:405–417. [PubMed] [Google Scholar]
- Mielke B., Möller P. Histomorphologic and immunophenotypic spectrum of primary gastro-intestinal B-cell lymphomas. Int J Cancer. 1991 Feb 1;47(3):334–343. doi: 10.1002/ijc.2910470304. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Allen T. D. Ultrastructure of cell loss in intestinal mucosa. J Ultrastruct Res. 1977 Aug;60(2):272–277. doi: 10.1016/s0022-5320(77)80071-3. [DOI] [PubMed] [Google Scholar]
- Potten C. S. Extreme sensitivity of some intestinal crypt cells to X and gamma irradiation. Nature. 1977 Oct 6;269(5628):518–521. doi: 10.1038/269518a0. [DOI] [PubMed] [Google Scholar]
- Potten C. S., Kellett M., Roberts S. A., Rew D. A., Wilson G. D. Measurement of in vivo proliferation in human colorectal mucosa using bromodeoxyuridine. Gut. 1992 Jan;33(1):71–78. doi: 10.1136/gut.33.1.71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potten C. S. Kinetics and possible regulation of crypt cell populations under normal and stress conditions. Bull Cancer. 1975 Oct-Dec;62(4):419–430. [PubMed] [Google Scholar]
- Potten C. S., Li Y. Q., O'Connor P. J., Winton D. J. A possible explanation for the differential cancer incidence in the intestine, based on distribution of the cytotoxic effects of carcinogens in the murine large bowel. Carcinogenesis. 1992 Dec;13(12):2305–2312. doi: 10.1093/carcin/13.12.2305. [DOI] [PubMed] [Google Scholar]
- Risio M., Coverlizza S., Ferrari A., Candelaresi G. L., Rossini F. P. Immunohistochemical study of epithelial cell proliferation in hyperplastic polyps, adenomas, and adenocarcinomas of the large bowel. Gastroenterology. 1988 Apr;94(4):899–906. doi: 10.1016/0016-5085(88)90545-8. [DOI] [PubMed] [Google Scholar]
- Roncucci L., Pedroni M., Fante R., Di Gregorio C., Ponz de Leon M. Cell kinetic evaluation of human colonic aberrant crypts. (Colorectal Cancer Study Group of the University of Modena and the Health Care District 16, Modena, Italy). Cancer Res. 1993 Aug 15;53(16):3726–3729. [PubMed] [Google Scholar]
- Rubio C. A., Alm T., Aly A., Poppen B. Intraepithelial bodies in colorectal adenomas: Leuchtenberger bodies revisited. Dis Colon Rectum. 1991 Jan;34(1):47–50. doi: 10.1007/BF02050206. [DOI] [PubMed] [Google Scholar]
- Searle J., Kerr J. F., Bishop C. J. Necrosis and apoptosis: distinct modes of cell death with fundamentally different significance. Pathol Annu. 1982;17(Pt 2):229–259. [PubMed] [Google Scholar]
- Sträter J., Günthert A. R., Brüderlein S., Möller P. Microwave irradiation of paraffin-embedded tissue sensitizes the TUNEL method for in situ detection of apoptotic cells. Histochem Cell Biol. 1995 Feb;103(2):157–160. doi: 10.1007/BF01454013. [DOI] [PubMed] [Google Scholar]
- Wyllie A. H., Kerr J. F., Currie A. R. Cell death: the significance of apoptosis. Int Rev Cytol. 1980;68:251–306. doi: 10.1016/s0074-7696(08)62312-8. [DOI] [PubMed] [Google Scholar]