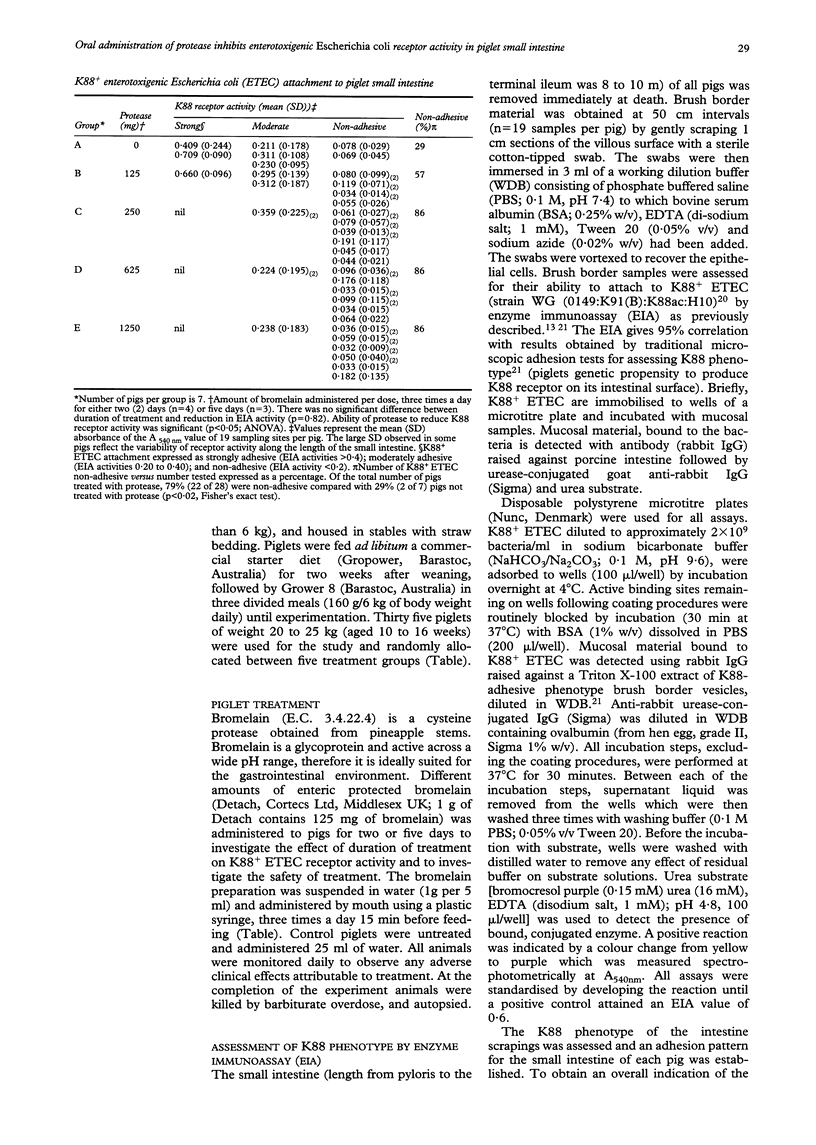
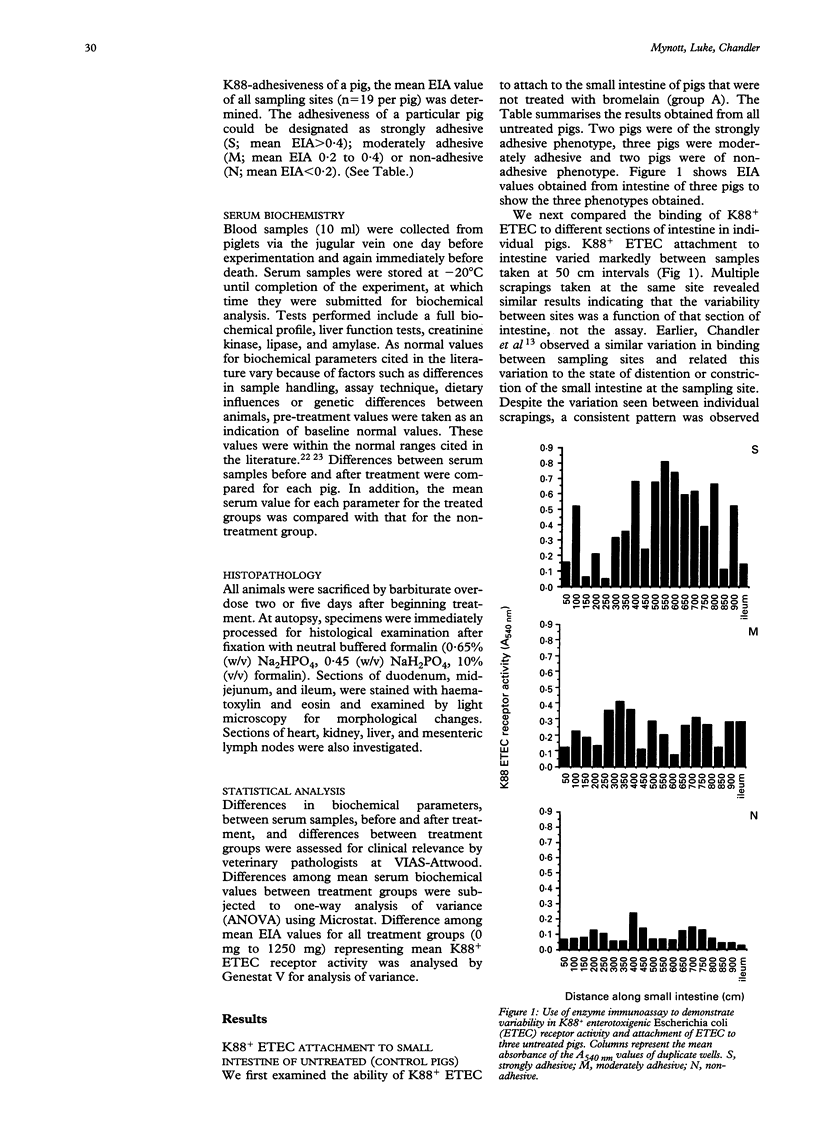
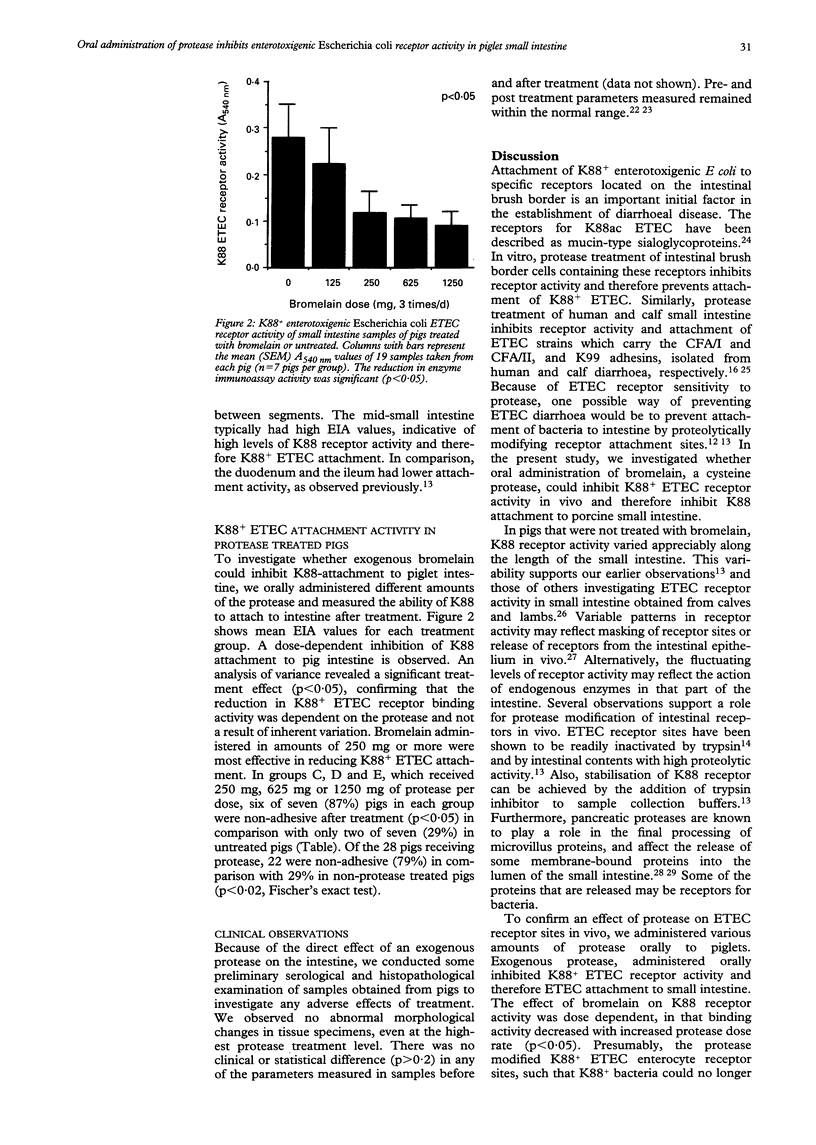
Abstract
The virulence of enterotoxigenic Escherichia coli (ETEC) is attributed to their ability to adhere via fimbrial adhesins to specific receptors located on the intestinal mucosa. A novel approach to preventing ETEC induced diarrhoea would be to prevent attachment of ETEC to intestine by proteolytically modifying the receptor attachment sites. This study aimed to examine the effect of bromelain, a proteolytic extract obtained from pineapple stems, on ETEC receptor activity in porcine small intestine. Bromelain was administered orally to piglets and K88+ ETEC attachment to small intestine was measured at 50 cm intervals using an enzyme immunoassay. K88+ ETEC attachment to intestinal sections that were not treated with bromelain varied appreciably between sampling sites. Variability in receptor activity along the intestinal surface is though to be caused by the localised effects of endogenous proteases. Oral administration of exogenous protease inhibited K88+ ETEC attachment to pig small intestine in a dose dependent manner (p < 0.05). Attachment of K88+ ETEC was negligible after treatment, resembling the levels of attachment of K88 to piglets of the genetically determined non-adhesive phenotype, which are resistant to K88+ ETEC infection. Serum biochemical analysis and histopathological examination of treated piglets showed no adverse effects of the bromelain treatment. It is concluded that administration of bromelain can inhibit ETEC receptor activity in vivo and may therefore be useful for prevention of K88+ ETEC induced diarrhoea.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bijlsma I. G., de Nijs A., van der Meer C., Frik J. F. Different pig phenotypes affect adherence of Escherichia coli to jejunal brush borders by K88ab, K88ac, or K88ad antigen. Infect Immun. 1982 Sep;37(3):891–894. doi: 10.1128/iai.37.3.891-894.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chandler D. S., Chandler H. M., Luke R. K., Tzipori S. R., Craven J. A. Screening of pig intestines for K88 non-adhesive phenotype by enzyme immunoassay. Vet Microbiol. 1986 Feb;11(1-2):153–161. doi: 10.1016/0378-1135(86)90015-5. [DOI] [PubMed] [Google Scholar]
- Chandler D. S., Mynott T. L., Luke R. K., Craven J. A. The distribution and stability of Escherichia coli K88 receptor in the gastrointestinal tract of the pig. Vet Microbiol. 1994 Jan;38(3):203–215. doi: 10.1016/0378-1135(94)90002-7. [DOI] [PubMed] [Google Scholar]
- Cox E., Houvenaghel A. In vitro adhesion of K88ab-, K88ac- and K88ad-positive Escherichia coli to intestinal villi, to buccal cells and to erythrocytes of weaned piglets. Vet Microbiol. 1987 Nov;15(3):201–207. doi: 10.1016/0378-1135(87)90074-5. [DOI] [PubMed] [Google Scholar]
- Cravioto A., Reyes R. E., Ortega R., Fernández G., Hernández R., López D. Prospective study of diarrhoeal disease in a cohort of rural Mexican children: incidence and isolated pathogens during the first two years of life. Epidemiol Infect. 1988 Aug;101(1):123–134. doi: 10.1017/s0950268800029289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dean E. A., Whipp S. C., Moon H. W. Age-specific colonization of porcine intestinal epithelium by 987P-piliated enterotoxigenic Escherichia coli. Infect Immun. 1989 Jan;57(1):82–87. doi: 10.1128/iai.57.1.82-87.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson A. K., Baker D. R., Bosworth B. T., Casey T. A., Benfield D. A., Francis D. H. Characterization of porcine intestinal receptors for the K88ac fimbrial adhesin of Escherichia coli as mucin-type sialoglycoproteins. Infect Immun. 1994 Dec;62(12):5404–5410. doi: 10.1128/iai.62.12.5404-5410.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaastra W., de Graaf F. K. Host-specific fimbrial adhesins of noninvasive enterotoxigenic Escherichia coli strains. Microbiol Rev. 1982 Jun;46(2):129–161. doi: 10.1128/mr.46.2.129-161.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hauri H. P., Quaroni A., Isselbacher K. J. Biogenesis of intestinal plasma membrane: posttranslational route and cleavage of sucrase-isomaltase. Proc Natl Acad Sci U S A. 1979 Oct;76(10):5183–5186. doi: 10.1073/pnas.76.10.5183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones G. W., Rutter J. M. Role of the K88 antigen in the pathogenesis of neonatal diarrhea caused by Escherichia coli in piglets. Infect Immun. 1972 Dec;6(6):918–927. doi: 10.1128/iai.6.6.918-927.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klemm P. Fimbrial adhesions of Escherichia coli. Rev Infect Dis. 1985 May-Jun;7(3):321–340. doi: 10.1093/clinids/7.3.321. [DOI] [PubMed] [Google Scholar]
- Laux D. C., McSweegan E. F., Williams T. J., Wadolkowski E. A., Cohen P. S. Identification and characterization of mouse small intestine mucosal receptors for Escherichia coli K-12(K88ab). Infect Immun. 1986 Apr;52(1):18–25. doi: 10.1128/iai.52.1.18-25.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe J. W., Krogfelt K. A., Krivan H. C., Cohen P. S., Laux D. C. Characterization and identification of a porcine small intestine mucus receptor for the K88ab fimbrial adhesin. Infect Immun. 1991 Jan;59(1):91–96. doi: 10.1128/iai.59.1.91-96.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouricout M. A., Julien R. A. Pilus-mediated binding of bovine enterotoxigenic Escherichia coli to calf small intestinal mucins. Infect Immun. 1987 May;55(5):1216–1223. doi: 10.1128/iai.55.5.1216-1223.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynott T. L., Chandler D. S., Luke R. K. Efficacy of enteric-coated protease in preventing attachment of enterotoxigenic Escherichia coli and diarrheal disease in the RITARD model. Infect Immun. 1991 Oct;59(10):3708–3714. doi: 10.1128/iai.59.10.3708-3714.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mynott T. L., Luke R. K., Chandler D. S. Detection of attachment of enterotoxigenic Escherichia coli (ETEC) to human small intestinal cells by enzyme immunoassay. FEMS Immunol Med Microbiol. 1995 Feb;10(3-4):207–218. doi: 10.1111/j.1574-695X.1995.tb00035.x. [DOI] [PubMed] [Google Scholar]
- Rutter J. M., Burrows M. R., Sellwood R., Gibbons R. A. A genetic basis for resistance to enteric disease caused by E. coli. Nature. 1975 Sep 11;257(5522):135–136. doi: 10.1038/257135a0. [DOI] [PubMed] [Google Scholar]
- Sellwood R., Gibbons R. A., Jones G. W., Rutter J. M. Adhesion of enteropathogenic Escherichia coli to pig intestinal brush borders: the existence of two pig phenotypes. J Med Microbiol. 1975 Aug;8(3):405–411. doi: 10.1099/00222615-8-3-405. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Linggood M. A. Further observations on Escherichia coli enterotoxins with particular regard to those produced by atypical piglet strains and by calf and lamb strains: the transmissible nature of these enterotoxins and of a K antigen possessed by calf and lamb strains. J Med Microbiol. 1972 May;5(2):243–250. doi: 10.1099/00222615-5-2-243. [DOI] [PubMed] [Google Scholar]
- Staley T. E., Wilson I. B. Soluble pig intestinal cell membrane components with affinities for E. coli K88+ antigen. Mol Cell Biochem. 1983;52(2):177–189. doi: 10.1007/BF00224926. [DOI] [PubMed] [Google Scholar]
- Tzipori S., Chandler D., Smith M., Makin T., Hennessy D. Factors contributing to postweaning diarrhoea in a large intensive piggery. Aust Vet J. 1980 Jun;56(6):274–278. doi: 10.1111/j.1751-0813.1980.tb05723.x. [DOI] [PubMed] [Google Scholar]


