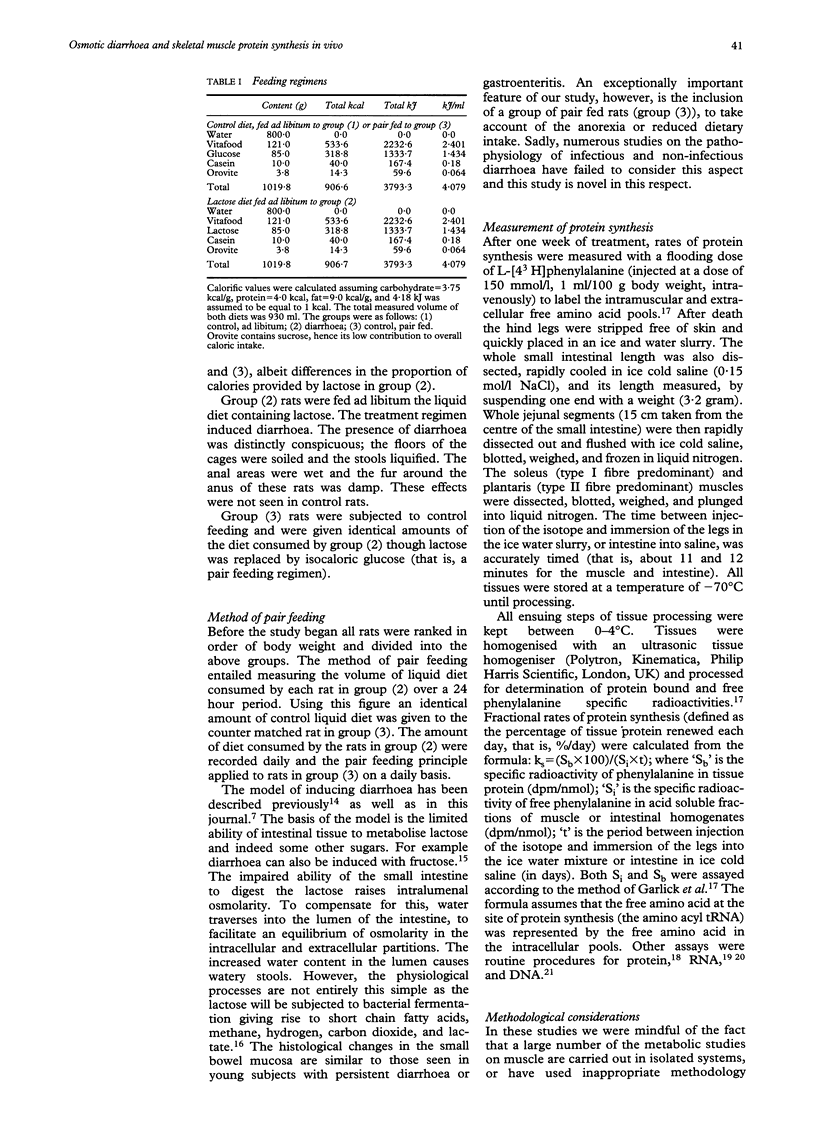
Abstract
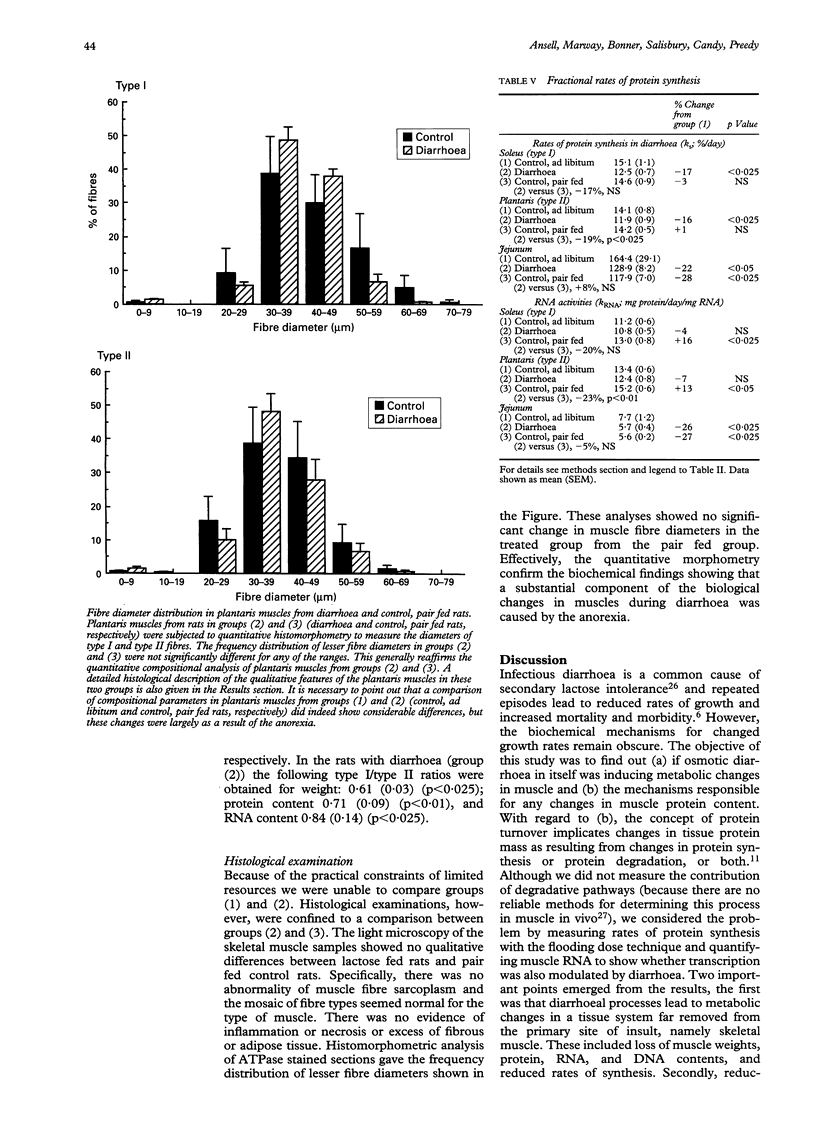
The pathogenic nature of the wasting seen in diarrhoea is unknown. This study measured protein synthesis in an established model of diarrhoea using lactose for seven days. Comparisons were also made with data obtained from rats fed an identical diet in which lactose was replaced by isocaloric glucose ad libitum (that is, the control diet). To account for diarrhoea induced anorexia, a third group of rats were included, which were fed identical amounts of the control diet as the rats with diarrhoea inducing diet. Comparisons of the diarrhoea induced group with rats fed the control diet ad libitum showed that diarrhoea caused a significant reduction in body weights. Type I and type II muscles showed significant reductions in protein, RNA, and DNA contents, as well as a fall in the derived parameters, RNA/DNA, protein/DNA, and RNA/protein. Fractional rates of protein synthesis (ks) were also reduced. However, synthesis rates of type I and II muscles relative to RNA (kRNA) were unchanged in these muscles in diarrhoea induced rats compared with ad libitum fed controls. In the jejunum there was an increase in the RNA/DNA ratio, and reductions in ks and kRNA. Comparisons were also made between rats with diarrhoea and rats pair fed the control diet. There were no changes in total muscle protein, RNA or DNA contents. This suggests that an important feature of body wasting in diarrhoea is the element of anorexia, which induces severe metabolic changes. The comparison between rats with diarrhoea and the pair fed group showed that histological features of the plantaris were not overtly changed, though diarrhoea caused significant reductions in RNA/DNA, protein/DNA, ks, and kRNA. Similar changes were seen for the soleus; though the reduction in ks failed to attain statistical significance. In the jejunum a comparison of diarrhoea induced rats with pair fed controls, showed increases in the ratios of RNA/DNA and protein/DNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ashford A. J., Pain V. M. Effect of diabetes on the rates of synthesis and degradation of ribosomes in rat muscle and liver in vivo. J Biol Chem. 1986 Mar 25;261(9):4059–4065. [PubMed] [Google Scholar]
- Brown K. H., Gastañaduy A. S., Saavedra J. M., Lembcke J., Rivas D., Robertson A. D., Yolken R., Sack R. B. Effect of continued oral feeding on clinical and nutritional outcomes of acute diarrhea in children. J Pediatr. 1988 Feb;112(2):191–200. doi: 10.1016/s0022-3476(88)80055-6. [DOI] [PubMed] [Google Scholar]
- Bruckstein A. H. Acute diarrhea. Am Fam Physician. 1988 Oct;38(4):217–228. [PubMed] [Google Scholar]
- Bueno J., Torres M., Almendros A., Carmona R., Nuñez M. C., Rios A., Gil A. Effect of dietary nucleotides on small intestinal repair after diarrhoea. Histological and ultrastructural changes. Gut. 1994 Jul;35(7):926–933. doi: 10.1136/gut.35.7.926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Collins J., Candy D. C., Starkey W. G., Spencer A. J., Osborne M. P., Stephen J. Disaccharidase activities in small intestine of rotavirus-infected suckling mice: a histochemical study. J Pediatr Gastroenterol Nutr. 1990 Oct;11(3):395–403. doi: 10.1097/00005176-199010000-00020. [DOI] [PubMed] [Google Scholar]
- Davidson G. P., Gall D. G., Petric M., Butler D. G., Hamilton J. R. Human rotavirus enteritis induced in conventional piglets. Intestinal structure and transport. J Clin Invest. 1977 Dec;60(6):1402–1409. doi: 10.1172/JCI108901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Downs T. R., Wilfinger W. W. Fluorometric quantification of DNA in cells and tissue. Anal Biochem. 1983 Jun;131(2):538–547. doi: 10.1016/0003-2697(83)90212-9. [DOI] [PubMed] [Google Scholar]
- Forget P., Sodoyez-Goffaux F., Zappitelli A. Permeability of the small intestine to [51Cr]EDTA in children with acute gastroenteritis or eczema. J Pediatr Gastroenterol Nutr. 1985 Jun;4(3):393–396. doi: 10.1097/00005176-198506000-00012. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Essén P., Wernerman J. Measurement of tissue protein synthesis rates in vivo: a critical analysis of contrasting methods. Am J Physiol. 1994 Mar;266(3 Pt 1):E287–E297. doi: 10.1152/ajpendo.1994.266.3.E287. [DOI] [PubMed] [Google Scholar]
- Garlick P. J., McNurlan M. A., Preedy V. R. A rapid and convenient technique for measuring the rate of protein synthesis in tissues by injection of [3H]phenylalanine. Biochem J. 1980 Nov 15;192(2):719–723. doi: 10.1042/bj1920719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goda T., Yamada K., Bustamante S., Edmond J., Grimes J., Koldovský O. Precocious increase of sucrase activity by carbohydrates in the small intestine of suckling rats. I. Significance of the stress effect of sugar-induced diarrhea. J Pediatr Gastroenterol Nutr. 1985 Jun;4(3):468–475. doi: 10.1097/00005176-198506000-00025. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Sackman J. W., Estes M. K. Pathogenesis of rotavirus-induced diarrhea. Preliminary studies in miniature swine piglet. Dig Dis Sci. 1984 Nov;29(11):1028–1035. doi: 10.1007/BF01311255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi M., Freiman D. G. An improved method of fixation for formalin-sensitive enzymes with special reference to myosin adenosine triphosphatase. J Histochem Cytochem. 1966 Aug;14(8):577–581. doi: 10.1177/14.8.577. [DOI] [PubMed] [Google Scholar]
- Holtug K., Clausen M. R., Hove H., Christiansen J., Mortensen P. B. The colon in carbohydrate malabsorption: short-chain fatty acids, pH, and osmotic diarrhoea. Scand J Gastroenterol. 1992 Jul;27(7):545–552. doi: 10.3109/00365529209000118. [DOI] [PubMed] [Google Scholar]
- Khoshoo V., Bhan M. K. Associated factors of protracted diarrhea. Indian Pediatr. 1990 Jun;27(6):559–569. [PubMed] [Google Scholar]
- Nuñez M. C., Ayudarte M. V., Morales D., Suarez M. D., Gil A. Effect of dietary nucleotides on intestinal repair in rats with experimental chronic diarrhea. JPEN J Parenter Enteral Nutr. 1990 Nov-Dec;14(6):598–604. doi: 10.1177/0148607190014006598. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. Factors affecting the activity of adenosine triphosphatase and other phosphatases as measured by histochemical techniques. J Histochem Cytochem. 1955 May;3(3):161–169. doi: 10.1177/3.3.161. [DOI] [PubMed] [Google Scholar]
- PADYKULA H. A., HERMAN E. The specificity of the histochemical method for adenosine triphosphatase. J Histochem Cytochem. 1955 May;3(3):170–195. doi: 10.1177/3.3.170. [DOI] [PubMed] [Google Scholar]
- Preedy V. R., Marway J. S., Peters T. J. Use of the Lieber-DeCarli liquid feeding regime with specific reference to the effects of ethanol on rat skeletal muscle RNA. Alcohol Alcohol. 1989;24(5):439–445. [PubMed] [Google Scholar]
- Preedy V. R., Siddiq T., Why H. J., Richardson P. J. Ethanol toxicity and cardiac protein synthesis in vivo. Am Heart J. 1994 May;127(5):1432–1439. doi: 10.1016/0002-8703(94)90072-8. [DOI] [PubMed] [Google Scholar]
- Rennie M. J., Smith K., Watt P. W. Measurement of human tissue protein synthesis: an optimal approach. Am J Physiol. 1994 Mar;266(3 Pt 1):E298–E307. doi: 10.1152/ajpendo.1994.266.3.E298. [DOI] [PubMed] [Google Scholar]
- Wapnir R. A., Litov R. E., Zdanowicz M. M., Lifshitz F. Improved water and sodium absorption from oral rehydration solutions based on rice syrup in a rat model of osmotic diarrhea. J Pediatr. 1991 Apr;118(4 Pt 2):S53–S61. doi: 10.1016/s0022-3476(05)81427-1. [DOI] [PubMed] [Google Scholar]


