Abstract
Neutrophils are important cellular mediators in inflammatory bowel disease (IBD). Interleukin (IL)8, a powerful neutrophil chemoattractant, is found in increased quantities in inflamed mucosa, but the cells of origin are uncertain. IL8 gene expression was studied by in situ hybridisation in uninflamed intestinal tissue resected for colon carcinoma (n = 7) and in inflamed colonic tissue resected for IBD (n = 11). Immunohistochemistry was used to assess the phenotype of IL8 expressing macrophages and the production of IL8 protein. Macrophages isolated from intestinal resections and lipopolysaccharide stimulated peripheral blood monocytes treated with 5-aminosalicylic acid, hydrocortisone, and cyclosporin A were examined for IL8 mRNA by northern blotting and IL8 secretion by enzyme linked immunosorbent assay (ELISA). In all cases IL8 mRNA was detected by in situ hybridisation in macrophages and neutrophils adjacent to ulceration in inflamed bowel, but not detected in uninflamed mucosa from carcinoma resections. Recently recruited CD14 positive macrophages were responsible for some of this IL8 expression. IL8 protein was present in the same distribution as mRNA. Epithelial cells in normal and inflamed tissue showed neither mRNA nor protein. IL8 mRNA was expressed significantly more commonly by macrophages from IBD affected than from normal mucosa, and IL8 secretion by IBD but not normal colon macrophages was augmented significantly by lipopolysaccharide treatment. IL8 expression and production by lipopolysaccharide treated blood monocytes was inhibited by the therapeutic agents tested. These results show that neutrophils and recently recruited macrophages are responsible for production of IL8 in IBD, suggesting a mechanism for a continuing cycle of neutrophil attraction. Agents used therapeutically in these diseases may be effective in part by disrupting this cycle.
Full text
PDF


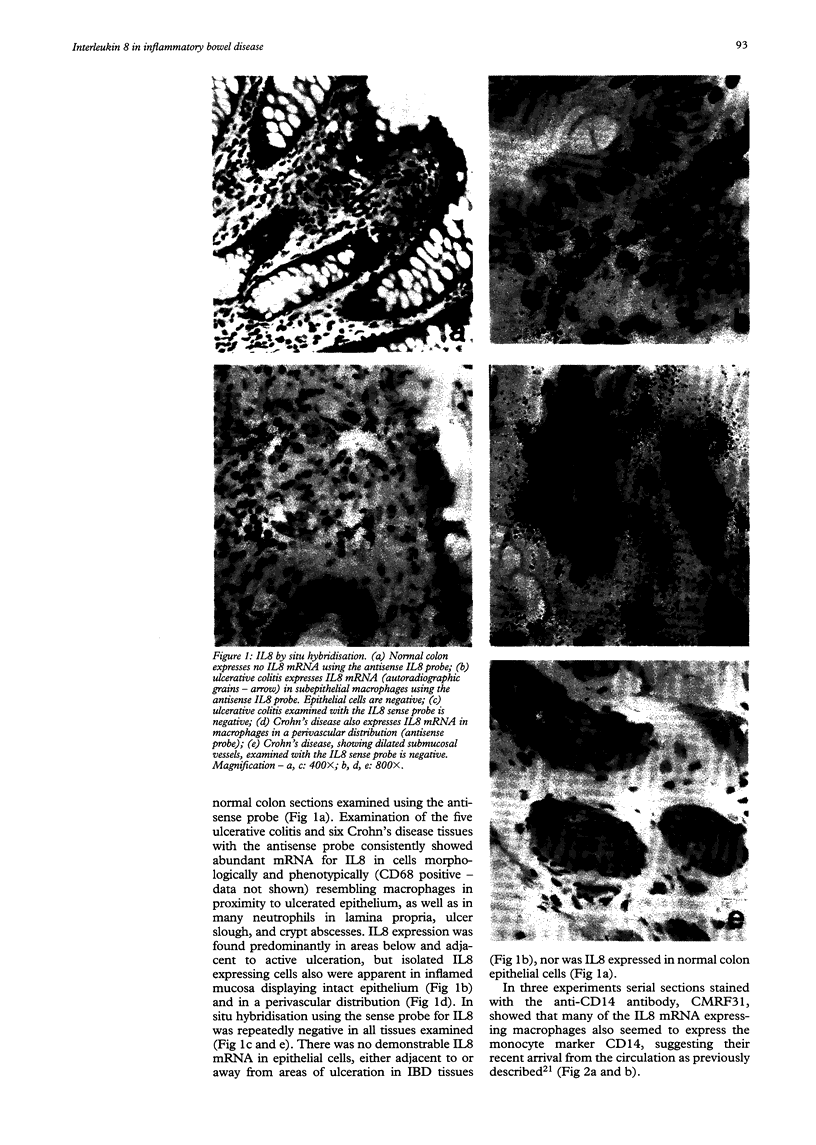





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anttila H. S., Reitamo S., Erkko P., Ceska M., Moser B., Baggiolini M. Interleukin-8 immunoreactivity in the skin of healthy subjects and patients with palmoplantar pustulosis and psoriasis. J Invest Dermatol. 1992 Jan;98(1):96–101. doi: 10.1111/1523-1747.ep12495817. [DOI] [PubMed] [Google Scholar]
- Birk P. E., Grimm P. C. Rapid nonradioactive in situ hybridization for interleukin-2 mRNA with riboprobes generated using the polymerase chain reaction. J Immunol Methods. 1994 Jan 3;167(1-2):83–89. doi: 10.1016/0022-1759(94)90077-9. [DOI] [PubMed] [Google Scholar]
- Cattoretti G., Pileri S., Parravicini C., Becker M. H., Poggi S., Bifulco C., Key G., D'Amato L., Sabattini E., Feudale E. Antigen unmasking on formalin-fixed, paraffin-embedded tissue sections. J Pathol. 1993 Oct;171(2):83–98. doi: 10.1002/path.1711710205. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cox K. H., DeLeon D. V., Angerer L. M., Angerer R. C. Detection of mrnas in sea urchin embryos by in situ hybridization using asymmetric RNA probes. Dev Biol. 1984 Feb;101(2):485–502. doi: 10.1016/0012-1606(84)90162-3. [DOI] [PubMed] [Google Scholar]
- Cunha F. Q., Ferreira S. H. The release of a neutrophil chemotactic factor from peritoneal macrophages by endotoxin: inhibition by glucocorticoids. Eur J Pharmacol. 1986 Sep 23;129(1-2):65–76. doi: 10.1016/0014-2999(86)90337-7. [DOI] [PubMed] [Google Scholar]
- Eckmann L., Jung H. C., Schürer-Maly C., Panja A., Morzycka-Wroblewska E., Kagnoff M. F. Differential cytokine expression by human intestinal epithelial cell lines: regulated expression of interleukin 8. Gastroenterology. 1993 Dec;105(6):1689–1697. doi: 10.1016/0016-5085(93)91064-o. [DOI] [PubMed] [Google Scholar]
- Eckmann L., Kagnoff M. F., Fierer J. Epithelial cells secrete the chemokine interleukin-8 in response to bacterial entry. Infect Immun. 1993 Nov;61(11):4569–4574. doi: 10.1128/iai.61.11.4569-4574.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golder J. P., Doe W. F. Isolation and preliminary characterization of human intestinal macrophages. Gastroenterology. 1983 Apr;84(4):795–802. [PubMed] [Google Scholar]
- Grimm M. C., Pavli P., Van de Pol E., Doe W. F. Evidence for a CD14+ population of monocytes in inflammatory bowel disease mucosa--implications for pathogenesis. Clin Exp Immunol. 1995 May;100(2):291–297. doi: 10.1111/j.1365-2249.1995.tb03667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grimm M. C., Pullman W. E., Bennett G. M., Sullivan P. J., Pavli P., Doe W. F. Direct evidence of monocyte recruitment to inflammatory bowel disease mucosa. J Gastroenterol Hepatol. 1995 Jul-Aug;10(4):387–395. doi: 10.1111/j.1440-1746.1995.tb01589.x. [DOI] [PubMed] [Google Scholar]
- Haziot A., Tsuberi B. Z., Goyert S. M. Neutrophil CD14: biochemical properties and role in the secretion of tumor necrosis factor-alpha in response to lipopolysaccharide. J Immunol. 1993 Jun 15;150(12):5556–5565. [PubMed] [Google Scholar]
- Isaacs K. L., Sartor R. B., Haskill S. Cytokine messenger RNA profiles in inflammatory bowel disease mucosa detected by polymerase chain reaction amplification. Gastroenterology. 1992 Nov;103(5):1587–1595. doi: 10.1016/0016-5085(92)91182-4. [DOI] [PubMed] [Google Scholar]
- Jones S. C., Evans S. W., Lobo A. J., Ceska M., Axon A. T., Whicher J. T. Serum interleukin-8 in inflammatory bowel disease. J Gastroenterol Hepatol. 1993 Nov-Dec;8(6):508–512. doi: 10.1111/j.1440-1746.1993.tb01643.x. [DOI] [PubMed] [Google Scholar]
- Koch A. E., Kunkel S. L., Pearce W. H., Shah M. R., Parikh D., Evanoff H. L., Haines G. K., Burdick M. D., Strieter R. M. Enhanced production of the chemotactic cytokines interleukin-8 and monocyte chemoattractant protein-1 in human abdominal aortic aneurysms. Am J Pathol. 1993 May;142(5):1423–1431. [PMC free article] [PubMed] [Google Scholar]
- Lewis R. A., Austen K. F., Soberman R. J. Leukotrienes and other products of the 5-lipoxygenase pathway. Biochemistry and relation to pathobiology in human diseases. N Engl J Med. 1990 Sep 6;323(10):645–655. doi: 10.1056/NEJM199009063231006. [DOI] [PubMed] [Google Scholar]
- Lloyd A. R., Oppenheim J. J. Poly's lament: the neglected role of the polymorphonuclear neutrophil in the afferent limb of the immune response. Immunol Today. 1992 May;13(5):169–172. doi: 10.1016/0167-5699(92)90121-M. [DOI] [PubMed] [Google Scholar]
- Mazzucchelli L., Hauser C., Zgraggen K., Wagner H., Hess M., Laissue J. A., Mueller C. Expression of interleukin-8 gene in inflammatory bowel disease is related to the histological grade of active inflammation. Am J Pathol. 1994 May;144(5):997–1007. [PMC free article] [PubMed] [Google Scholar]
- McCormick B. A., Colgan S. P., Delp-Archer C., Miller S. I., Madara J. L. Salmonella typhimurium attachment to human intestinal epithelial monolayers: transcellular signalling to subepithelial neutrophils. J Cell Biol. 1993 Nov;123(4):895–907. doi: 10.1083/jcb.123.4.895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean I. W., Nakane P. K. Periodate-lysine-paraformaldehyde fixative. A new fixation for immunoelectron microscopy. J Histochem Cytochem. 1974 Dec;22(12):1077–1083. doi: 10.1177/22.12.1077. [DOI] [PubMed] [Google Scholar]
- Oppenheim J. J., Zachariae C. O., Mukaida N., Matsushima K. Properties of the novel proinflammatory supergene "intercrine" cytokine family. Annu Rev Immunol. 1991;9:617–648. doi: 10.1146/annurev.iy.09.040191.003153. [DOI] [PubMed] [Google Scholar]
- Pavli P., Hume D. A., Van De Pol E., Doe W. F. Dendritic cells, the major antigen-presenting cells of the human colonic lamina propria. Immunology. 1993 Jan;78(1):132–141. [PMC free article] [PubMed] [Google Scholar]
- Podolsky D. K. Inflammatory bowel disease (1) N Engl J Med. 1991 Sep 26;325(13):928–937. doi: 10.1056/NEJM199109263251306. [DOI] [PubMed] [Google Scholar]
- Pullman W. E., Elsbury S., Kobayashi M., Hapel A. J., Doe W. F. Enhanced mucosal cytokine production in inflammatory bowel disease. Gastroenterology. 1992 Feb;102(2):529–537. doi: 10.1016/0016-5085(92)90100-d. [DOI] [PubMed] [Google Scholar]
- Pullman W. E., Sullivan P. J., Barratt P. J., Lising J., Booth J. A., Doe W. F. Assessment of inflammatory bowel disease activity by technetium 99m phagocyte scanning. Gastroenterology. 1988 Oct;95(4):989–996. doi: 10.1016/0016-5085(88)90174-6. [DOI] [PubMed] [Google Scholar]
- Pyke C., Kristensen P., Ralfkiaer E., Grøndahl-Hansen J., Eriksen J., Blasi F., Danø K. Urokinase-type plasminogen activator is expressed in stromal cells and its receptor in cancer cells at invasive foci in human colon adenocarcinomas. Am J Pathol. 1991 May;138(5):1059–1067. [PMC free article] [PubMed] [Google Scholar]
- Raab Y., Gerdin B., Ahlstedt S., Hällgren R. Neutrophil mucosal involvement is accompanied by enhanced local production of interleukin-8 in ulcerative colitis. Gut. 1993 Sep;34(9):1203–1206. doi: 10.1136/gut.34.9.1203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuerer-Maly C. C., Eckmann L., Kagnoff M. F., Falco M. T., Maly F. E. Colonic epithelial cell lines as a source of interleukin-8: stimulation by inflammatory cytokines and bacterial lipopolysaccharide. Immunology. 1994 Jan;81(1):85–91. [PMC free article] [PubMed] [Google Scholar]
- Seitz M., Dewald B., Gerber N., Baggiolini M. Enhanced production of neutrophil-activating peptide-1/interleukin-8 in rheumatoid arthritis. J Clin Invest. 1991 Feb;87(2):463–469. doi: 10.1172/JCI115018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobhani I., Hochlaf S., Denizot Y., Vissuzaine C., Rene E., Benveniste J., Lewin M. M., Mignon M. Raised concentrations of platelet activating factor in colonic mucosa of Crohn's disease patients. Gut. 1992 Sep;33(9):1220–1225. doi: 10.1136/gut.33.9.1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stephens R. W., Golder J. P., Fayle D. R., Hume D. A., Hapel A. J., Allan W., Fordham C. J., Doe W. F. Minactivin expression in human monocyte and macrophage populations. Blood. 1985 Aug;66(2):333–337. [PubMed] [Google Scholar]
- Taub D. D., Oppenheim J. J. Chemokines, inflammation and the immune system. Ther Immunol. 1994 Aug;1(4):229–246. [PubMed] [Google Scholar]
- Van Zee K. J., DeForge L. E., Fischer E., Marano M. A., Kenney J. S., Remick D. G., Lowry S. F., Moldawer L. L. IL-8 in septic shock, endotoxemia, and after IL-1 administration. J Immunol. 1991 May 15;146(10):3478–3482. [PubMed] [Google Scholar]
- Weiss S. J. Tissue destruction by neutrophils. N Engl J Med. 1989 Feb 9;320(6):365–376. doi: 10.1056/NEJM198902093200606. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Ramos R. A., Tobias P. S., Ulevitch R. J., Mathison J. C. CD14, a receptor for complexes of lipopolysaccharide (LPS) and LPS binding protein. Science. 1990 Sep 21;249(4975):1431–1433. doi: 10.1126/science.1698311. [DOI] [PubMed] [Google Scholar]
- Ziegler-Heitbrock H. W., Pechumer H., Petersmann I., Durieux J. J., Vita N., Labeta M. O., Ströbel M. CD14 is expressed and functional in human B cells. Eur J Immunol. 1994 Aug;24(8):1937–1940. doi: 10.1002/eji.1830240835. [DOI] [PubMed] [Google Scholar]
- Zipfel P. F., Bialonski A., Skerka C. Induction of members of the IL-8/NAP-1 gene family in human T lymphocytes is suppressed by cyclosporin A. Biochem Biophys Res Commun. 1991 Nov 27;181(1):179–183. doi: 10.1016/s0006-291x(05)81398-1. [DOI] [PubMed] [Google Scholar]






