Abstract
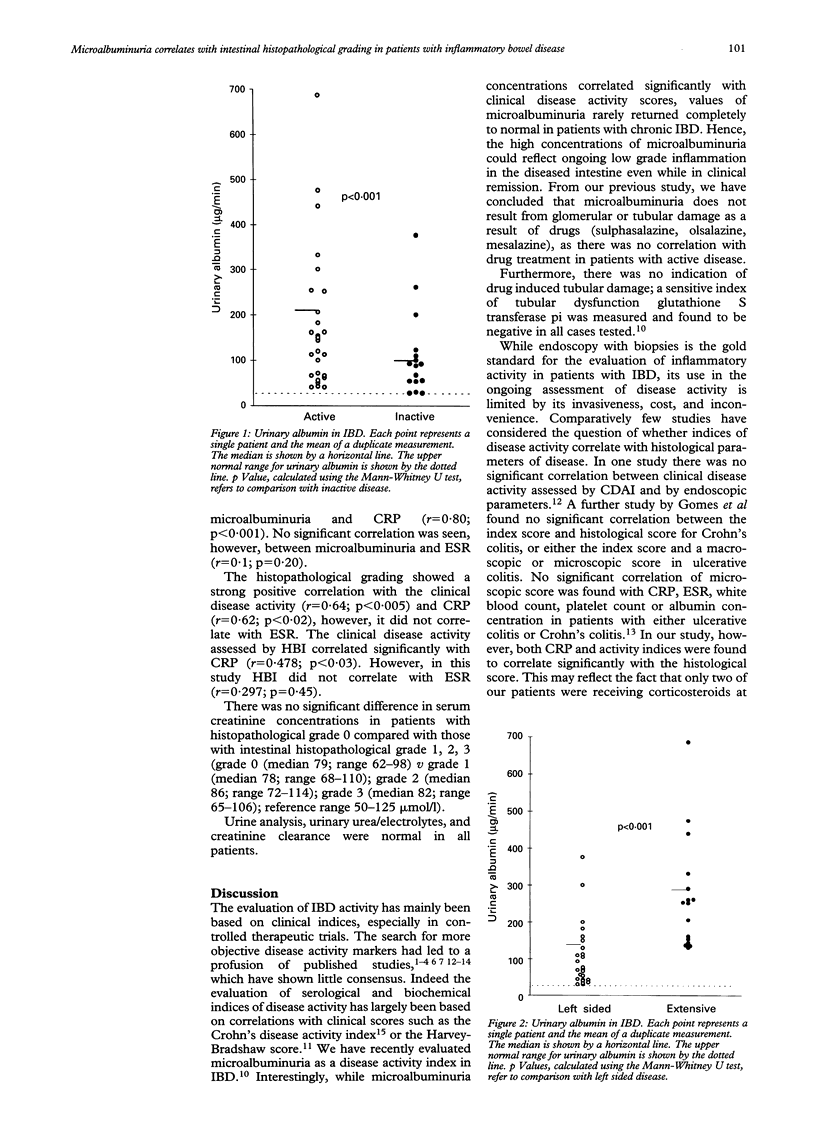
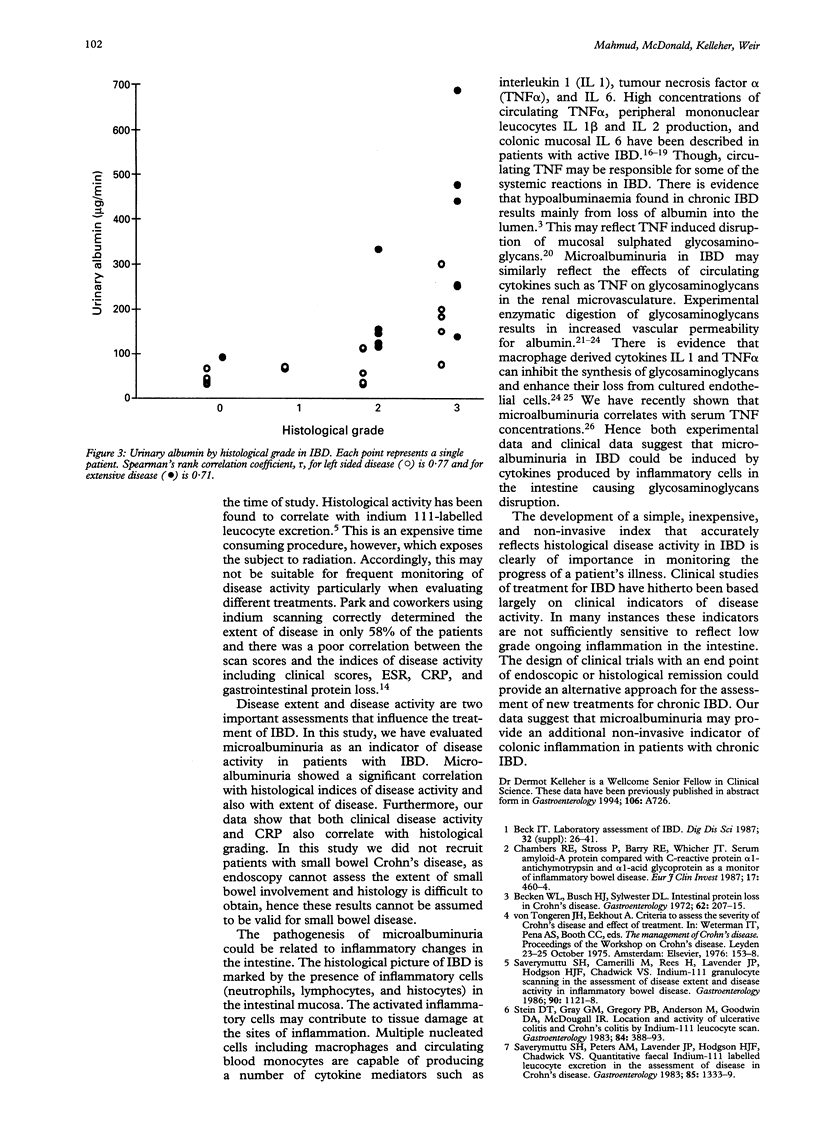
It has previously been shown that microalbuminuria is a useful disease activity marker for inflammatory bowel disease (IBD). Microalbuminuria correlates strongly with the markers of clinical and laboratory disease activity such as erythrocyte sedimentation rate (ESR), and C reactive protein (CRP). The aim of this study was to discover if microalbuminuria accurately reflects the intestinal inflammation by correlating it with intestinal inflammation using a standard histopathological grading system in patients with ulcerative colitis and Crohn's colitis. Forty two patients with IBD who had undergone endoscopic examination of the entire colon for the assessment of severity and extent of the disease (Crohn's colitis (n = 21), ulcerative colitis (n = 21)) were recruited to the study. Patients with small bowel Crohn's disease were not studied. Twenty four patients had left sided colonic disease and 18 patients had extensive colonic disease. Each patient's colonic biopsy specimens were scored blindly by a histopathologist and a composite score was compiled on the basis of the severity of changes in the enterocytes and crypts and the cellularity of the lamina propria. A clinical disease activity was obtained using the simple index of Harvey and Bradshaw. Microalbuminuria was measured in all patients by an immunoturbiditimetric method. ESR and CRP were also measured, as indicators of acute phase response in the same patients. It was found that patients with active IBD had higher concentrations of microalbuminuria compared with those patients in remission (median 222 micrograms/min (range 40-686 micrograms/min) v median 96 micrograms/min (range 30-376 micrograms/min); p < 0.001)). Significantly higher concentrations of microalbuminuria were also detected in patients with extensive colonic IBD compared with those patients with left sided disease (median 297 micrograms/min (range 132-686 micrograms/min) v median 101 micrograms/min (range 30-433 micrograms/min); p < 0.001)). A strong positive correlation was seen between microalbuminuria and intestinal histopathological score in IBD patient groups with left sided colitis (r = 0.77; p < 0.001) and extensive disease (r = 0.71; p < 0.01). The standard histopathological grading system correlated with the clinical disease activity (r = 0.64; p < 0.005) and CRP (r = 0.62; p < 0.02), however, it did not correlate with ESR. In conclusion, the strong correlation of microalbuminuria with a standard intestinal histopathological grading system suggests that microalbuminuria accurately reflects the severity of colonic inflammation in patients with Crohn's colitis and ulcerative colitis.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beeken W. L., Busch H. J., Sylwester D. L. Intestinal protein loss in Crohn's disease. Gastroenterology. 1972 Feb;62(2):207–215. [PubMed] [Google Scholar]
- Best W. R., Becktel J. M., Singleton J. W., Kern F., Jr Development of a Crohn's disease activity index. National Cooperative Crohn's Disease Study. Gastroenterology. 1976 Mar;70(3):439–444. [PubMed] [Google Scholar]
- Cellier C., Sahmoud T., Froguel E., Adenis A., Belaiche J., Bretagne J. F., Florent C., Bouvry M., Mary J. Y., Modigliani R. Correlations between clinical activity, endoscopic severity, and biological parameters in colonic or ileocolonic Crohn's disease. A prospective multicentre study of 121 cases. The Groupe d'Etudes Thérapeutiques des Affections Inflammatoires Digestives. Gut. 1994 Feb;35(2):231–235. doi: 10.1136/gut.35.2.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chambers R. E., Stross P., Barry R. E., Whicher J. T. Serum amyloid A protein compared with C-reactive protein, alpha 1-antichymotrypsin and alpha 1-acid glycoprotein as a monitor of inflammatory bowel disease. Eur J Clin Invest. 1987 Oct;17(5):460–467. doi: 10.1111/j.1365-2362.1987.tb01143.x. [DOI] [PubMed] [Google Scholar]
- Harvey R. F., Bradshaw J. M. A simple index of Crohn's-disease activity. Lancet. 1980 Mar 8;1(8167):514–514. doi: 10.1016/s0140-6736(80)92767-1. [DOI] [PubMed] [Google Scholar]
- Kanwar Y. S., Linker A., Farquhar M. G. Increased permeability of the glomerular basement membrane to ferritin after removal of glycosaminoglycans (heparan sulfate) by enzyme digestion. J Cell Biol. 1980 Aug;86(2):688–693. doi: 10.1083/jcb.86.2.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein N. J., Shennan G. I., Heyderman R. S., Levin M. Alteration in glycosaminoglycan metabolism and surface charge on human umbilical vein endothelial cells induced by cytokines, endotoxin and neutrophils. J Cell Sci. 1992 Aug;102(Pt 4):821–832. doi: 10.1242/jcs.102.4.821. [DOI] [PubMed] [Google Scholar]
- Krentz A. J., Hale P. J., Albutt E. C., Nattrass M. HbA1 in the diagnosis of factitious remission of diabetes. Ann Clin Biochem. 1988 Mar;25(Pt 2):150–154. doi: 10.1177/000456328802500204. [DOI] [PubMed] [Google Scholar]
- Mahmud N., O'Connell M. A., Stinson J., Goggins M. G., Weir D. G., Kelleher D. Tumour necrosis factor-alpha and microalbuminuria in patients with inflammatory bowel disease. Eur J Gastroenterol Hepatol. 1995 Mar;7(3):215–219. [PubMed] [Google Scholar]
- Mahmud N., Stinson J., O'Connell M. A., Mantle T. J., Keeling P. W., Feely J., Weir D. G., Kelleher D. Microalbuminuria in inflammatory bowel disease. Gut. 1994 Nov;35(11):1599–1604. doi: 10.1136/gut.35.11.1599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitsuyama K., Sasaki E., Toyonaga A., Ikeda H., Tsuruta O., Irie A., Arima N., Oriishi T., Harada K., Fujisaki K. Colonic mucosal interleukin-6 in inflammatory bowel disease. Digestion. 1991;50(2):104–111. doi: 10.1159/000200747. [DOI] [PubMed] [Google Scholar]
- Murch S. H., Lamkin V. A., Savage M. O., Walker-Smith J. A., MacDonald T. T. Serum concentrations of tumour necrosis factor alpha in childhood chronic inflammatory bowel disease. Gut. 1991 Aug;32(8):913–917. doi: 10.1136/gut.32.8.913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murch S. H., MacDonald T. T., Walker-Smith J. A., Levin M., Lionetti P., Klein N. J. Disruption of sulphated glycosaminoglycans in intestinal inflammation. Lancet. 1993 Mar 20;341(8847):711–714. doi: 10.1016/0140-6736(93)90485-y. [DOI] [PubMed] [Google Scholar]
- Nakamura M., Saito H., Kasanuki J., Tamura Y., Yoshida S. Cytokine production in patients with inflammatory bowel disease. Gut. 1992 Jul;33(7):933–937. doi: 10.1136/gut.33.7.933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathan C. F. Secretory products of macrophages. J Clin Invest. 1987 Feb;79(2):319–326. doi: 10.1172/JCI112815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park R. H., McKillop J. H., Duncan A., MacKenzie J. F., Russell R. I. Can 111indium autologous mixed leucocyte scanning accurately assess disease extent and activity in Crohn's disease? Gut. 1988 Jun;29(6):821–825. doi: 10.1136/gut.29.6.821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig L. J., Kanwar Y. S. Removal of sulfated (heparan sulfate) or nonsulfated (hyaluronic acid) glycosaminoglycans results in increased permeability of the glomerular basement membrane to 125I-bovine serum albumin. Lab Invest. 1982 Aug;47(2):177–184. [PubMed] [Google Scholar]
- Saverymuttu S. H., Camilleri M., Rees H., Lavender J. P., Hodgson H. J., Chadwick V. S. Indium 111-granulocyte scanning in the assessment of disease extent and disease activity in inflammatory bowel disease. A comparison with colonoscopy, histology, and fecal indium 111-granulocyte excretion. Gastroenterology. 1986 May;90(5 Pt 1):1121–1128. doi: 10.1016/0016-5085(86)90376-8. [DOI] [PubMed] [Google Scholar]
- Saverymuttu S. H., Peters A. M., Lavender J. P., Pepys M. B., Hodgson H. J., Chadwick V. S. Quantitative fecal indium 111-labeled leukocyte excretion in the assessment of disease in Crohn's disease. Gastroenterology. 1983 Dec;85(6):1333–1339. [PubMed] [Google Scholar]
- Stein D. T., Gray G. M., Gregory P. B., Anderson M., Goodwin D. A., McDougall I. R. Location and activity of ulcerative and Crohn's colitis by indium 111 leukocyte scan. A prospective comparison study. Gastroenterology. 1983 Feb;84(2):388–393. [PubMed] [Google Scholar]
- Vehaskari V. M., Chang C. T., Stevens J. K., Robson A. M. The effects of polycations on vascular permeability in the rat. A proposed role for charge sites. J Clin Invest. 1984 Apr;73(4):1053–1061. doi: 10.1172/JCI111290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yi E. S., Ulich T. R. Endotoxin, interleukin-1, and tumor necrosis factor cause neutrophil-dependent microvascular leakage in postcapillary venules. Am J Pathol. 1992 Mar;140(3):659–663. [PMC free article] [PubMed] [Google Scholar]



