Abstract
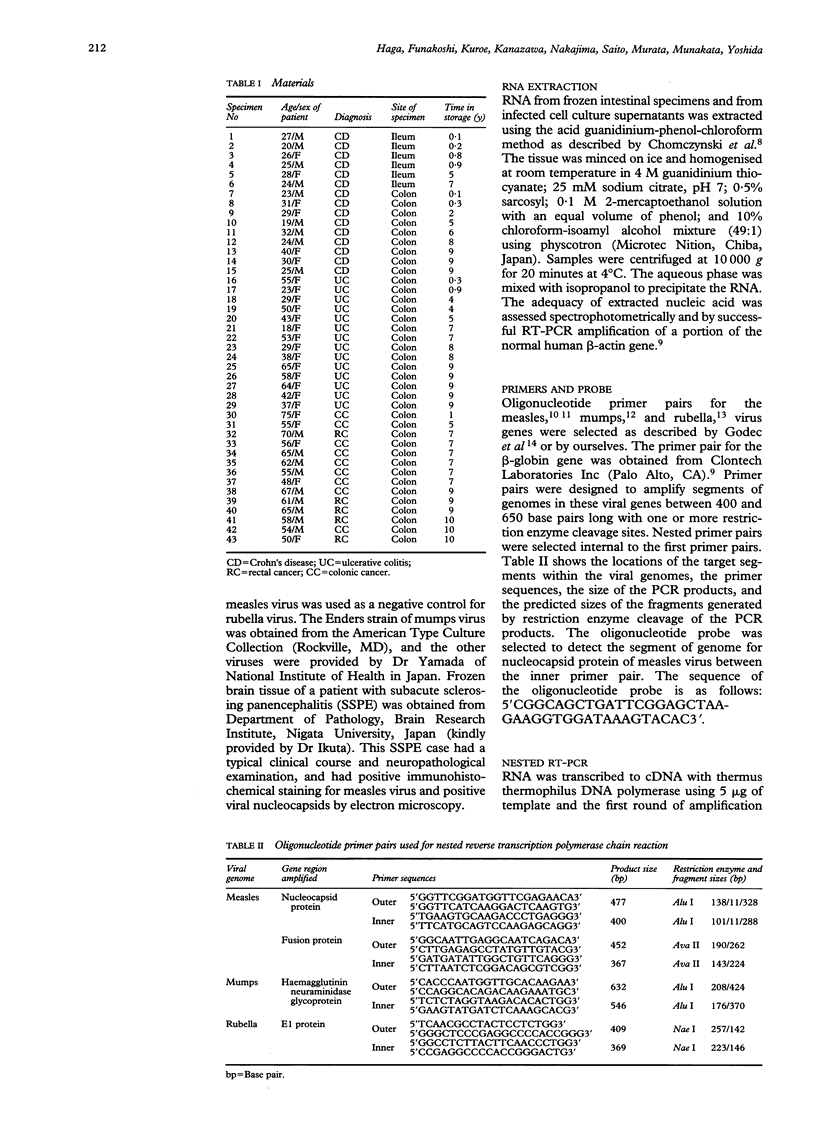
The aetiology of Crohn's disease remains unknown, although evidence for a viral cause has long been sought. Recent studies have shown inflammation of the submucosal microvascular endothelium and granulomata, and endothelial cell cytoplasmic inclusions, consistent with paramyxovirus, were identified by electron microscopy suggesting a persistent measles virus infection in Crohn's disease. Measles, mumps, and rubella viruses were tested for Crohn's disease by polymerase chain reaction (PCR). RNA was extracted from resected intestinal specimens from 15 patients with Crohn's disease, 14 with ulcerative colitis, and 14 controls without inflammatory bowel disease. This was used to perform nested PCR after reverse transcription (RT) of the RNA to cDNA with primer pairs directed against two regions in the genome of the measles virus and one region in the mumps and rubella viral genomes. Despite enhanced sensitivity of nested RT-PCR, measles, mumps, and rubella viral genomic sequences were not found in any intestinal specimen.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckland R., Gerald C., Barker R., Wild T. F. Fusion glycoprotein of measles virus: nucleotide sequence of the gene and comparison with other paramyxoviruses. J Gen Virol. 1987 Jun;68(Pt 6):1695–1703. doi: 10.1099/0022-1317-68-6-1695. [DOI] [PubMed] [Google Scholar]
- Chiodini R. J., Van Kruiningen H. J., Merkal R. S., Thayer W. R., Jr, Coutu J. A. Characteristics of an unclassified Mycobacterium species isolated from patients with Crohn's disease. J Clin Microbiol. 1984 Nov;20(5):966–971. doi: 10.1128/jcm.20.5.966-971.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Fidler H. M., Rook G. A., Johnson N. M., McFadden J. Mycobacterium tuberculosis DNA in tissue affected by sarcoidosis. BMJ. 1993 Feb 27;306(6877):546–549. doi: 10.1136/bmj.306.6877.546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gitnick G. L., Rosen V. J. Electron microscopic studies of viral agents in Crohn's disease. Lancet. 1976 Jul 31;2(7979):217–219. doi: 10.1016/s0140-6736(76)91023-0. [DOI] [PubMed] [Google Scholar]
- Godec M. S., Asher D. M., Murray R. S., Shin M. L., Greenham L. W., Gibbs C. J., Jr, Gajdusek D. C. Absence of measles, mumps, and rubella viral genomic sequences from multiple sclerosis brain tissue by polymerase chain reaction. Ann Neurol. 1992 Sep;32(3):401–404. doi: 10.1002/ana.410320317. [DOI] [PubMed] [Google Scholar]
- Kwok S., Higuchi R. Avoiding false positives with PCR. Nature. 1989 May 18;339(6221):237–238. doi: 10.1038/339237a0. [DOI] [PubMed] [Google Scholar]
- Li H. H., Gyllensten U. B., Cui X. F., Saiki R. K., Erlich H. A., Arnheim N. Amplification and analysis of DNA sequences in single human sperm and diploid cells. Nature. 1988 Sep 29;335(6189):414–417. doi: 10.1038/335414a0. [DOI] [PubMed] [Google Scholar]
- Mitchell D. N., Rees R. J. Agent transmissible from Crohn's disease tissue. Lancet. 1970 Jul 25;2(7665):168–171. doi: 10.1016/s0140-6736(70)92532-8. [DOI] [PubMed] [Google Scholar]
- Mullis K. B., Faloona F. A. Specific synthesis of DNA in vitro via a polymerase-catalyzed chain reaction. Methods Enzymol. 1987;155:335–350. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- Nakajima-Iijima S., Hamada H., Reddy P., Kakunaga T. Molecular structure of the human cytoplasmic beta-actin gene: interspecies homology of sequences in the introns. Proc Natl Acad Sci U S A. 1985 Sep;82(18):6133–6137. doi: 10.1073/pnas.82.18.6133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakhasi H. L., Meyer B. C., Liu T. Y. Rubella virus cDNA. Sequence and expression of E1 envelope protein. J Biol Chem. 1986 Dec 15;261(35):16616–16621. [PubMed] [Google Scholar]
- Rozenblatt S., Eizenberg O., Ben-Levy R., Lavie V., Bellini W. J. Sequence homology within the morbilliviruses. J Virol. 1985 Feb;53(2):684–690. doi: 10.1128/jvi.53.2.684-690.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Saiki R. K., Scharf S., Faloona F., Mullis K. B., Horn G. T., Erlich H. A., Arnheim N. Enzymatic amplification of beta-globin genomic sequences and restriction site analysis for diagnosis of sickle cell anemia. Science. 1985 Dec 20;230(4732):1350–1354. doi: 10.1126/science.2999980. [DOI] [PubMed] [Google Scholar]
- Veres G., Gibbs R. A., Scherer S. E., Caskey C. T. The molecular basis of the sparse fur mouse mutation. Science. 1987 Jul 24;237(4813):415–417. doi: 10.1126/science.3603027. [DOI] [PubMed] [Google Scholar]
- Wakefield A. J., Pittilo R. M., Sim R., Cosby S. L., Stephenson J. R., Dhillon A. P., Pounder R. E. Evidence of persistent measles virus infection in Crohn's disease. J Med Virol. 1993 Apr;39(4):345–353. doi: 10.1002/jmv.1890390415. [DOI] [PubMed] [Google Scholar]
- Wakefield A. J., Sankey E. A., Dhillon A. P., Sawyerr A. M., More L., Sim R., Pittilo R. M., Rowles P. M., Hudson M., Lewis A. A. Granulomatous vasculitis in Crohn's disease. Gastroenterology. 1991 May;100(5 Pt 1):1279–1287. [PubMed] [Google Scholar]
- Wakefield A. J., Sawyerr A. M., Dhillon A. P., Pittilo R. M., Rowles P. M., Lewis A. A., Pounder R. E. Pathogenesis of Crohn's disease: multifocal gastrointestinal infarction. Lancet. 1989 Nov 4;2(8671):1057–1062. doi: 10.1016/s0140-6736(89)91078-7. [DOI] [PubMed] [Google Scholar]
- Waxham M. N., Aronowski J., Server A. C., Wolinsky J. S., Smith J. A., Goodman H. M. Sequence determination of the mumps virus HN gene. Virology. 1988 Jun;164(2):318–325. doi: 10.1016/0042-6822(88)90544-2. [DOI] [PubMed] [Google Scholar]
- Whorwell P. J., Phillips C. A., Beeken W. L., Little P. K., Roessner K. D. Isolation of reovirus-like agents from patients with Crohn's disease. Lancet. 1977 Jun 4;1(8023):1169–1171. doi: 10.1016/s0140-6736(77)92714-3. [DOI] [PubMed] [Google Scholar]




