Abstract
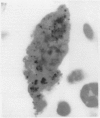
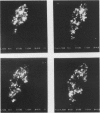
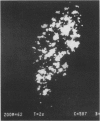
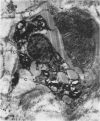
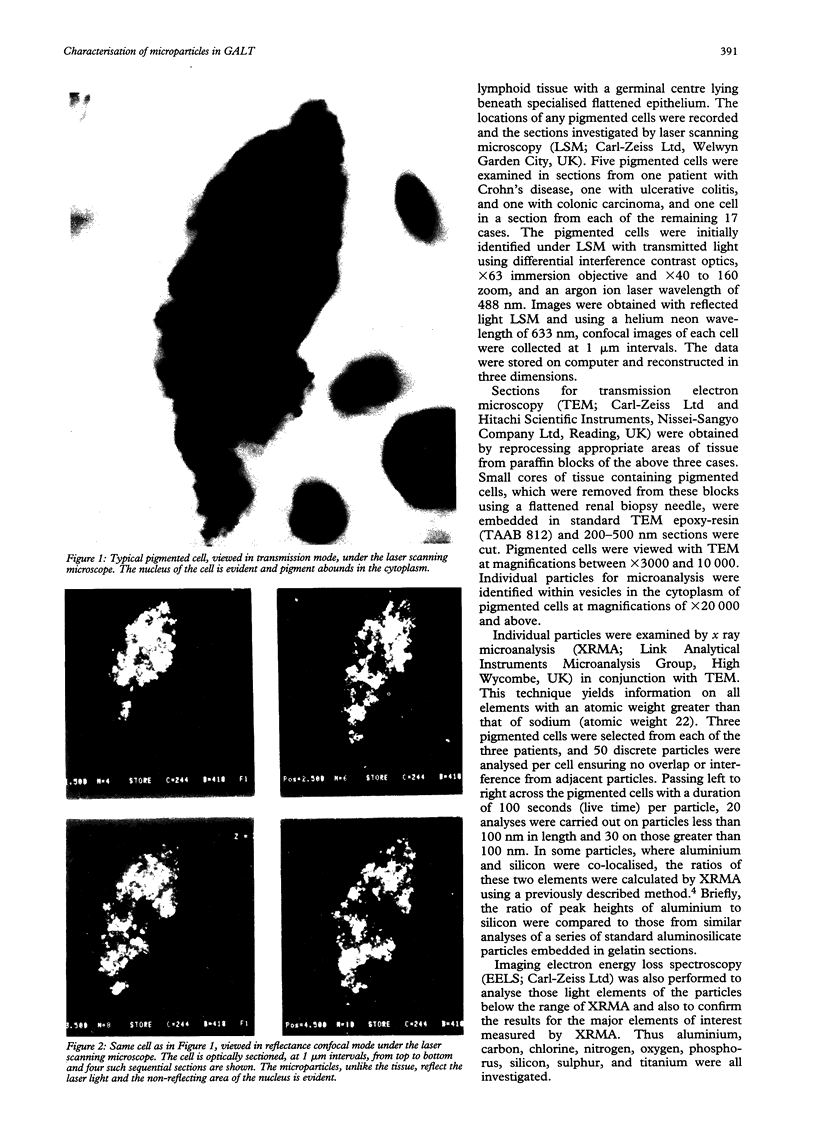
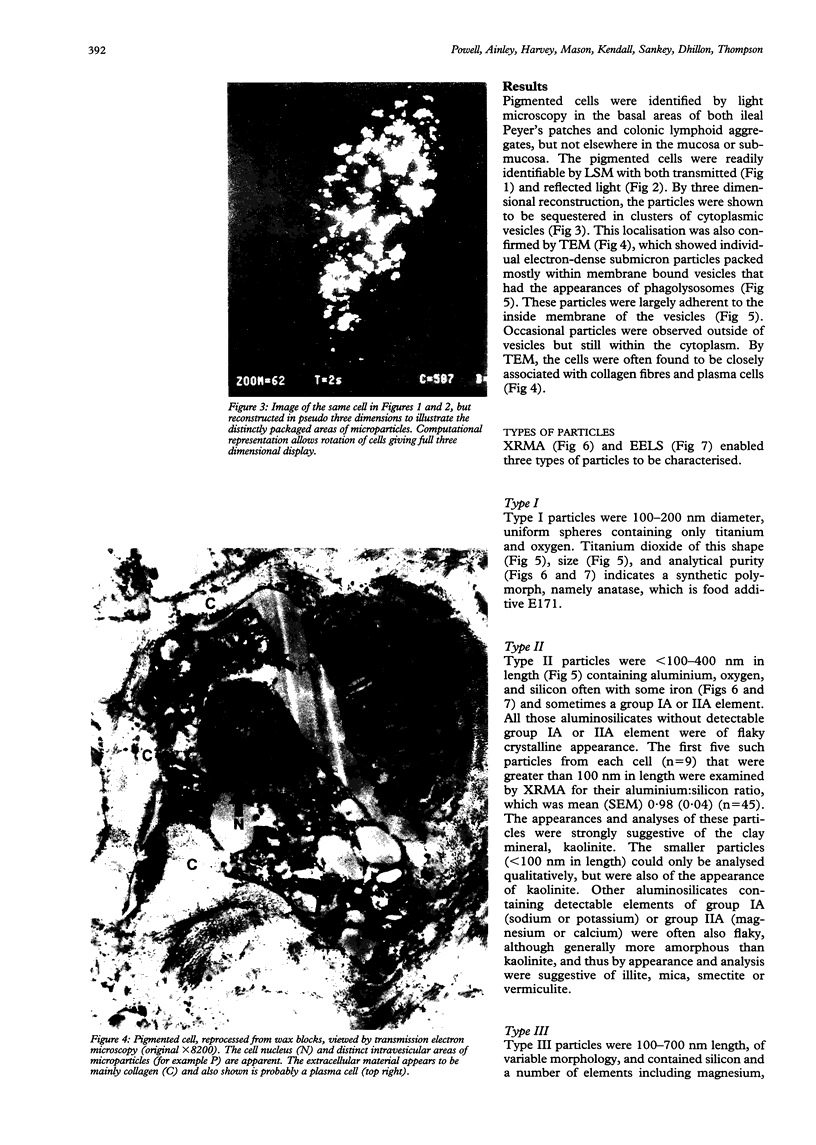
Macrophages at the base of human gut associated lymphoid tissue (GALT), become loaded early in life with dark granular pigment that is rich in aluminium, silicon, and titanium. The molecular characteristics, intracellular distribution, and source of this pigment is described. Laser scanning and electron microscopy showed that pigmented macrophages were often closely related to collagen fibres and plasma cells in GALT of both small and large intestine and contained numerous phagolysosomes, previously described as granules, that are rich in electron dense submicron sized particles. Morphological assessment, x ray microanalysis, and image electron energy loss spectroscopy showed three distinct types of microparticle: type I - spheres of titanium dioxide, 100-200 nm diameter, characterised as the synthetic food-additive polymorph anatase; type II - aluminosilicates, < 100-400 nm in length, generally of flaky appearance, often with adsorbed surface iron, and mostly characteristic of the natural clay mineral kaolinite; and type III - mixed environmental silicates without aluminium, 100-700 nm in length and of variable morphology. Thus, this cellular pigment that is partly derived from food additives and partly from the environment is composed of inert inorganic microparticles and loaded into phagolysosomes of macrophages within the GALT of all human subjects. These observations suggest that the pathogenicity of this pigment should be further investigated since, in susceptible individuals, the same intracellular distribution of these three types of submicron particle causes chronic latent granulomatous inflammation.
Full text
PDF

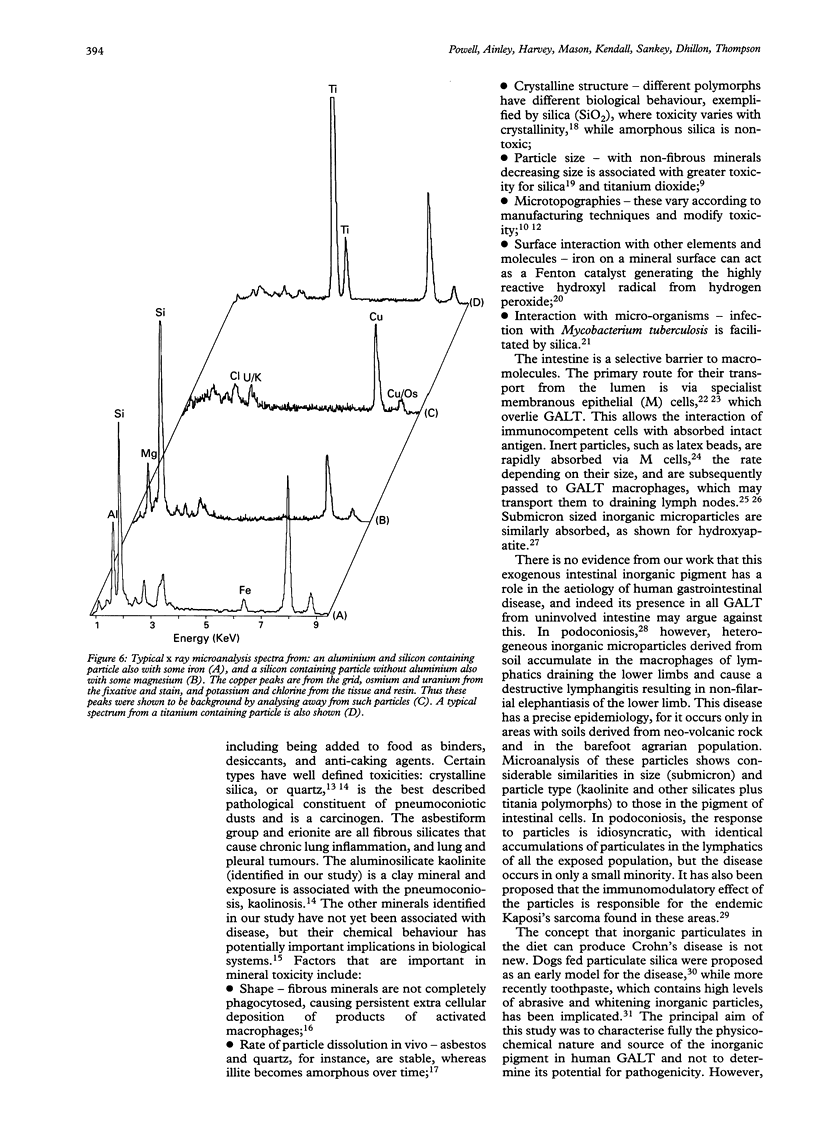
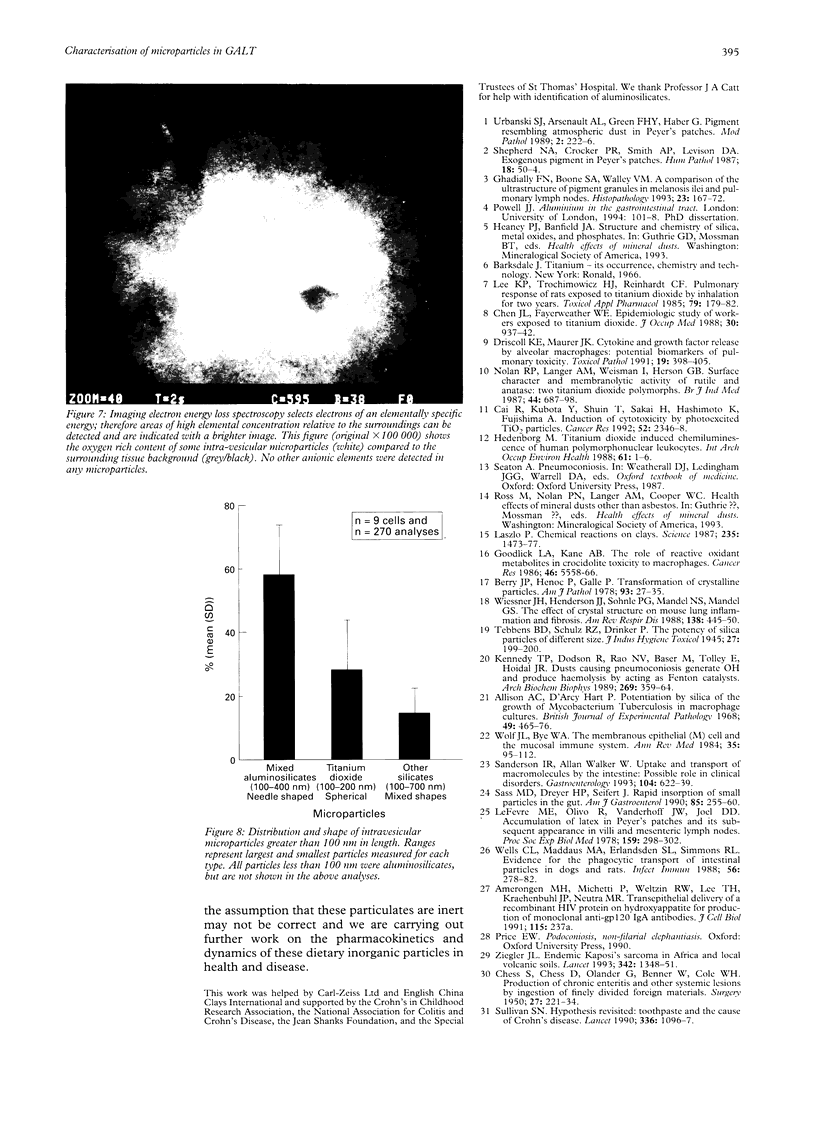
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allison A. C., Hart P. D. Potentiation by silica of the growth of Mycobacterium tuberculosis in macrophage cultures. Br J Exp Pathol. 1968 Oct;49(5):465–476. [PMC free article] [PubMed] [Google Scholar]
- Cai R., Kubota Y., Shuin T., Sakai H., Hashimoto K., Fujishima A. Induction of cytotoxicity by photoexcited TiO2 particles. Cancer Res. 1992 Apr 15;52(8):2346–2348. [PubMed] [Google Scholar]
- Driscoll K. E., Maurer J. K. Cytokine and growth factor release by alveolar macrophages: potential biomarkers of pulmonary toxicity. Toxicol Pathol. 1991;19(4 Pt 1):398–405. doi: 10.1177/0192623391019004-108. [DOI] [PubMed] [Google Scholar]
- Ghadially F. N., Boone S. A., Walley V. M. A comparison of the ultrastructure of pigment granules in melanosis ilei and pulmonary lymph nodes. Histopathology. 1993 Aug;23(2):167–172. doi: 10.1111/j.1365-2559.1993.tb00475.x. [DOI] [PubMed] [Google Scholar]
- Goodglick L. A., Kane A. B. Role of reactive oxygen metabolites in crocidolite asbestos toxicity to mouse macrophages. Cancer Res. 1986 Nov;46(11):5558–5566. [PubMed] [Google Scholar]
- Kennedy T. P., Dodson R., Rao N. V., Ky H., Hopkins C., Baser M., Tolley E., Hoidal J. R. Dusts causing pneumoconiosis generate .OH and produce hemolysis by acting as Fenton catalysts. Arch Biochem Biophys. 1989 Feb 15;269(1):359–364. doi: 10.1016/0003-9861(89)90118-5. [DOI] [PubMed] [Google Scholar]
- LeFevre M. E., Olivo R., Vanderhoff J. W., Joel D. D. Accumulation of latex in Peyer's patches and its subsequent appearance in villi and mesenteric lymph nodes. Proc Soc Exp Biol Med. 1978 Nov;159(2):298–302. doi: 10.3181/00379727-159-40336. [DOI] [PubMed] [Google Scholar]
- Lee K. P., Trochimowicz H. J., Reinhardt C. F. Pulmonary response of rats exposed to titanium dioxide (TiO2) by inhalation for two years. Toxicol Appl Pharmacol. 1985 Jun 30;79(2):179–192. doi: 10.1016/0041-008x(85)90339-4. [DOI] [PubMed] [Google Scholar]
- Nolan R. P., Langer A. M., Weisman I., Herson G. B. Surface character and membranolytic activity of rutile and anatase: two titanium dioxide polymorphs. Br J Ind Med. 1987 Oct;44(10):687–698. doi: 10.1136/oem.44.10.687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanderson I. R., Walker W. A. Uptake and transport of macromolecules by the intestine: possible role in clinical disorders (an update). Gastroenterology. 1993 Feb;104(2):622–639. doi: 10.1016/0016-5085(93)90436-g. [DOI] [PubMed] [Google Scholar]
- Sass W., Dreyer H. P., Seifert J. Rapid insorption of small particles in the gut. Am J Gastroenterol. 1990 Mar;85(3):255–260. [PubMed] [Google Scholar]
- Shepherd N. A., Crocker P. R., Smith A. P., Levison D. A. Exogenous pigment in Peyer's patches. Hum Pathol. 1987 Jan;18(1):50–54. doi: 10.1016/s0046-8177(87)80193-4. [DOI] [PubMed] [Google Scholar]
- Sullivan S. N. Hypothesis revisited: toothpaste and the cause of Crohn's disease. Lancet. 1990 Nov 3;336(8723):1096–1097. doi: 10.1016/0140-6736(90)92572-y. [DOI] [PubMed] [Google Scholar]
- Urbanski S. J., Arsenault A. L., Green F. H., Haber G. Pigment resembling atmospheric dust in Peyer's patches. Mod Pathol. 1989 May;2(3):222–226. [PubMed] [Google Scholar]
- Wells C. L., Maddaus M. A., Erlandsen S. L., Simmons R. L. Evidence for the phagocytic transport of intestinal particles in dogs and rats. Infect Immun. 1988 Jan;56(1):278–282. doi: 10.1128/iai.56.1.278-282.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiessner J. H., Henderson J. D., Jr, Sohnle P. G., Mandel N. S., Mandel G. S. The effect of crystal structure on mouse lung inflammation and fibrosis. Am Rev Respir Dis. 1988 Aug;138(2):445–450. doi: 10.1164/ajrccm/138.2.445. [DOI] [PubMed] [Google Scholar]
- Wolf J. L., Bye W. A. The membranous epithelial (M) cell and the mucosal immune system. Annu Rev Med. 1984;35:95–112. doi: 10.1146/annurev.me.35.020184.000523. [DOI] [PubMed] [Google Scholar]
- Ziegler J. L. Endemic Kaposi's sarcoma in Africa and local volcanic soils. Lancet. 1993 Nov 27;342(8883):1348–1351. doi: 10.1016/0140-6736(93)92252-o. [DOI] [PubMed] [Google Scholar]