Abstract
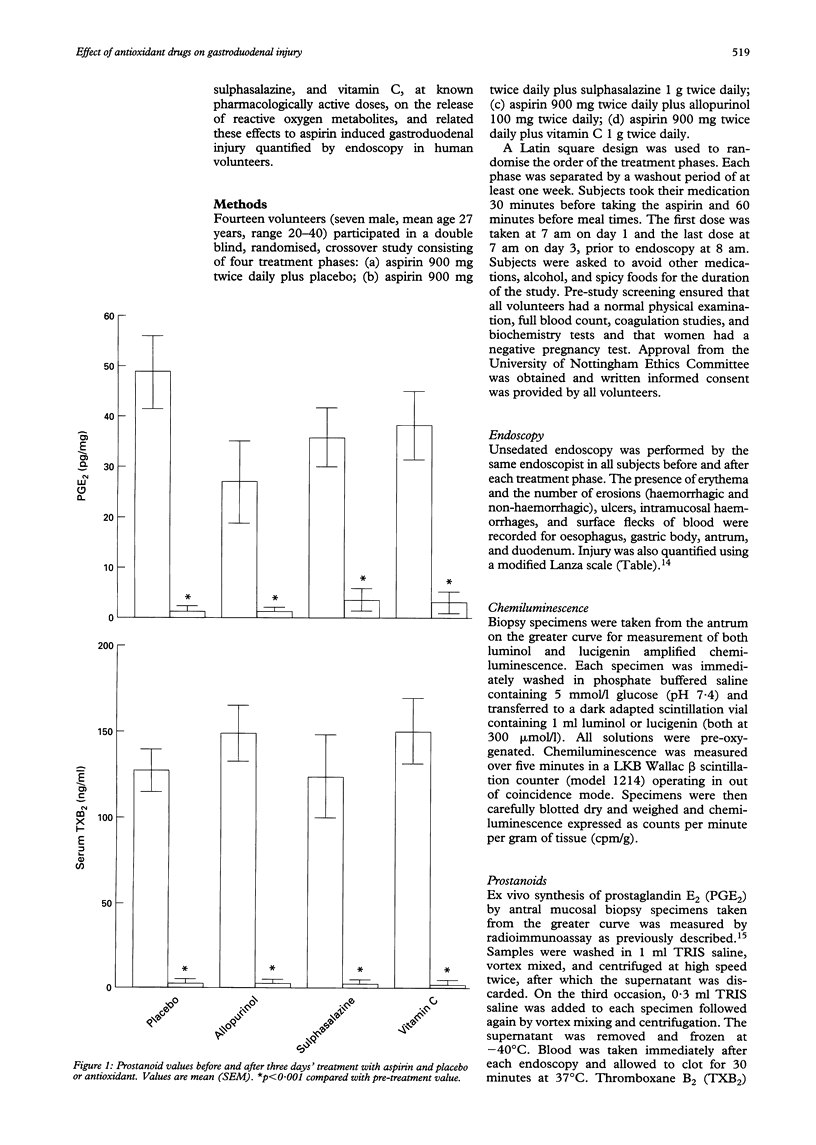
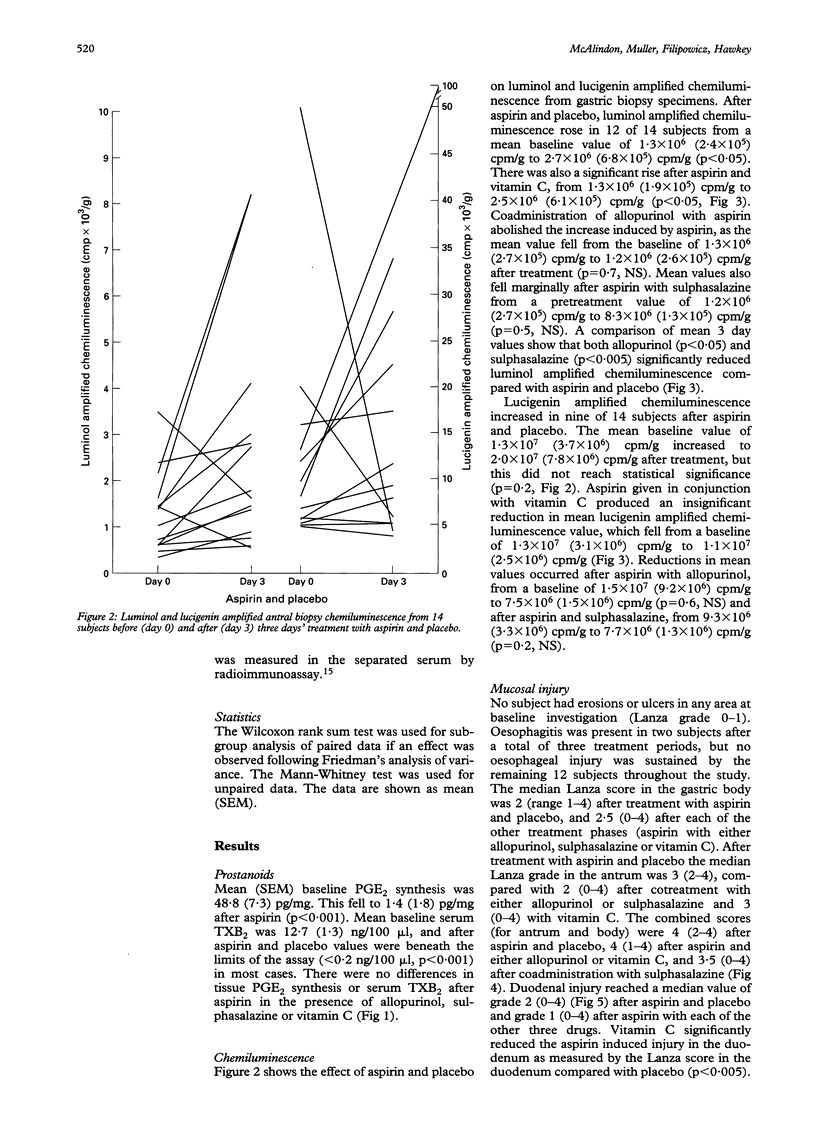
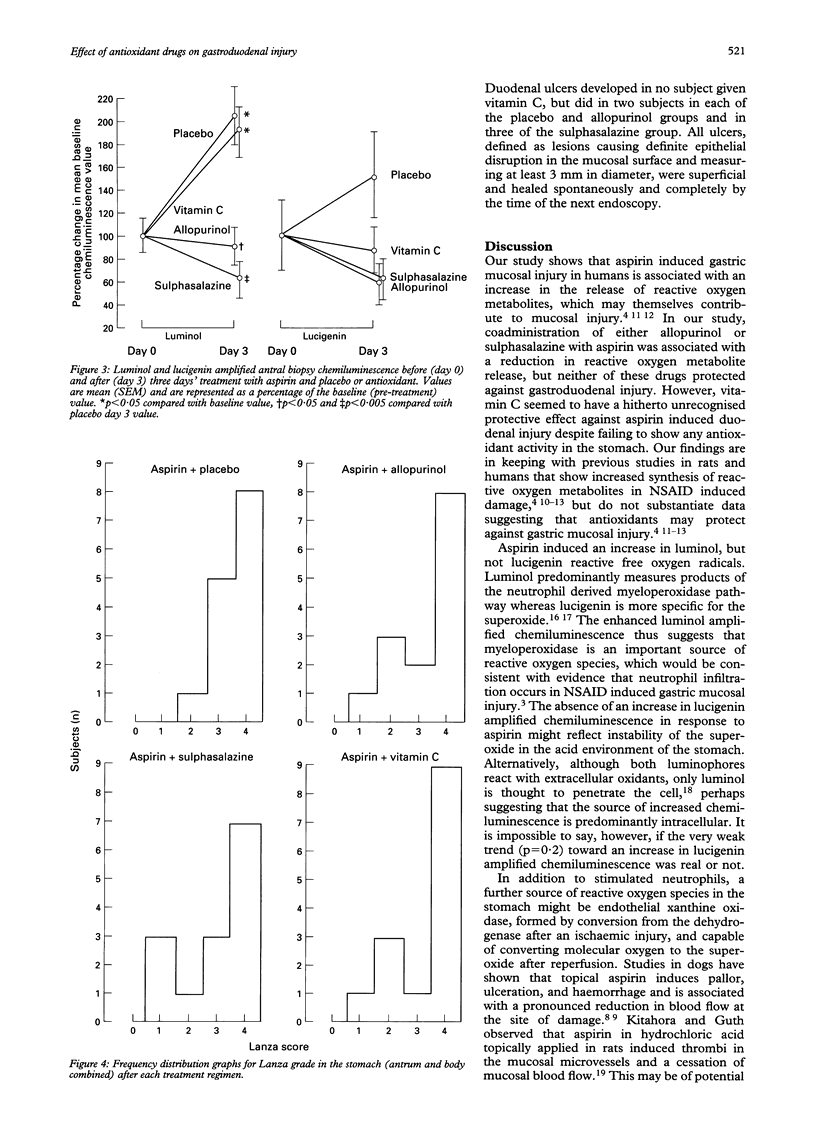
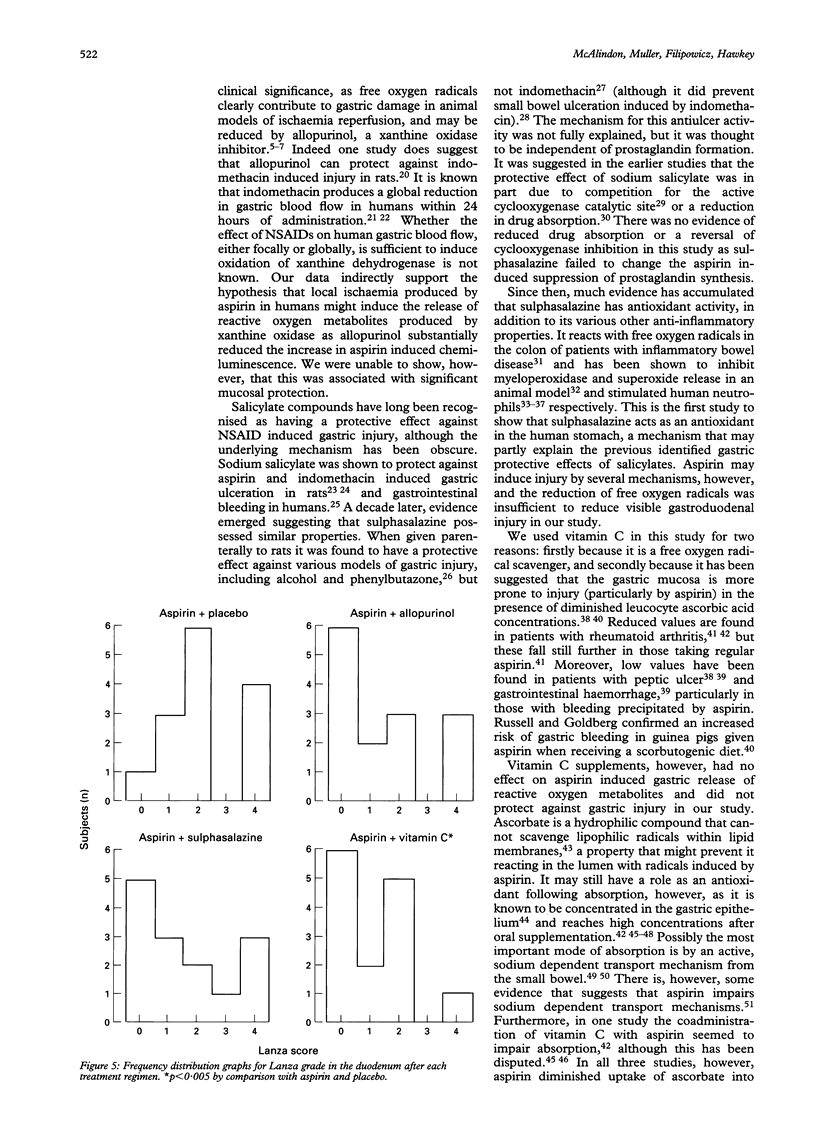
BACKGROUND--The mechanisms of aspirin induced gastroduodenal injury are not fully understood. Aspirin induces the release of reactive oxygen metabolites in animal models, which may contribute to mucosal injury. AIMS--To investigate the effects of aspirin administered with placebo or antioxidants on gastric mucosal reactive oxygen metabolite release and gastroduodenal injury in human volunteers. SUBJECTS--Fourteen healthy volunteers participated in the study (seven male; mean age 27 years, range 20-40). METHODS--In a double blind, randomised, crossover study, volunteers received aspirin 900 mg twice daily and either placebo, allopurinol 100 mg twice daily, sulphasalazine 1 g twice daily or vitamin C 1 g twice daily for three days. Injury was assessed endoscopically and by quantifying mucosal reactive oxygen metabolite release by measuring chemiluminescence before and after each treatment. The effect on prostanoids was determined by measuring ex vivo antral prostaglandin E2 (PGE2) synthesis and serum thromboxane B2 (TXB2). RESULTS--No drug reduced any parameter of gastric injury but vitamin C reduced duodenal injury assessed by Lanza score (p < 0.005). Chemiluminescence increased after aspirin both with placebo (p < 0.05) and vitamin C (p < 0.05). Post-treatment chemiluminescence was lower in subjects taking allopurinol (p < 0.05) or sulphasalazine (p < 0.005) than in those taking placebo with aspirin. CONCLUSIONS--In this study, aspirin induced gastric injury was associated with reactive oxygen metabolite release. This was reduced by sulphasalazine and allopurinol, although macroscopic injury was not affected. Vitamin C, however, was shown to have a previously unrecognised protective effect against aspirin induced duodenal injury.
Full text
PDF


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Afifi A. M., Ellis L., Huntsman R. G., Said M. I. High dose ascorbic acid in the management of thalassaemia leg ulcers--a pilot study. Br J Dermatol. 1975 Mar;92(3):339–341. doi: 10.1111/j.1365-2133.1975.tb03085.x. [DOI] [PubMed] [Google Scholar]
- Ahnfelt-Rønne I., Nielsen O. H., Christensen A., Langholz E., Binder V., Riis P. Clinical evidence supporting the radical scavenger mechanism of 5-aminosalicylic acid. Gastroenterology. 1990 May;98(5 Pt 1):1162–1169. doi: 10.1016/0016-5085(90)90329-y. [DOI] [PubMed] [Google Scholar]
- Allgayer H., Rang S., Klotz U., Böhne P., Retey J., Kruis W., Gugler R. Superoxide inhibition following different stimuli of respiratory burst and metabolism of aminosalicylates in neutrophils. Dig Dis Sci. 1994 Jan;39(1):145–151. doi: 10.1007/BF02090074. [DOI] [PubMed] [Google Scholar]
- Arvanitakis C., Chen G. H., Folscroft J., Greenberger N. J. Effect of aspirin on intestinal absorption of glucose, sodium, and water in man. Gut. 1977 Mar;18(3):187–190. doi: 10.1136/gut.18.3.187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ashley S. W., Sonnenschein L. A., Cheung L. Y. Focal gastric mucosal blood flow at the site of aspirin-induced ulceration. Am J Surg. 1985 Jan;149(1):53–59. doi: 10.1016/s0002-9610(85)80009-x. [DOI] [PubMed] [Google Scholar]
- Berry C. N., Prouteau M., Lloyd K. G. Sulphasalazine and PhCL28A inhibit the formation of ethanol- and phenylbutazone-induced rat gastric ulcers: lack of involvement of endogenous prostaglandins? Br J Pharmacol. 1988 Mar;93(3):465–472. doi: 10.1111/j.1476-5381.1988.tb10300.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bianchi J., Wilson F. A., Rose R. C. Dehydroascorbic acid and ascorbic acid transport systems in the guinea pig ileum. Am J Physiol. 1986 Apr;250(4 Pt 1):G461–G468. doi: 10.1152/ajpgi.1986.250.4.G461. [DOI] [PubMed] [Google Scholar]
- Cho C. H., Ogle C. W., Sevilla E. L. The protective effects of sulphasalazine against ethanol-induced gastric damage in rats. Br J Pharmacol. 1987 Sep;92(1):31–37. doi: 10.1111/j.1476-5381.1987.tb11292.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christiansen N. O. A time-course study on superoxide generation and protein kinase C activation in human neutrophils. FEBS Lett. 1988 Nov 7;239(2):195–198. doi: 10.1016/0014-5793(88)80915-3. [DOI] [PubMed] [Google Scholar]
- Cohen M. M., Duncan A. M. Ascorbic acid nutrition in gastroduodenal disorders. Br Med J. 1967 Dec 2;4(5578):516–518. doi: 10.1136/bmj.4.5578.516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeChatelet L. R., Long G. D., Shirley P. S., Bass D. A., Thomas M. J., Henderson F. W., Cohen M. S. Mechanism of the luminol-dependent chemiluminescence of human neutrophils. J Immunol. 1982 Oct;129(4):1589–1593. [PubMed] [Google Scholar]
- Del Soldato P., Foschi D., Varin L., Daniotti S. Indomethacin-induced intestinal ulcers in rats: effects of salicylazosulfapyridine and dexamethasone. Agents Actions. 1985 Jul;16(5):393–396. doi: 10.1007/BF01982878. [DOI] [PubMed] [Google Scholar]
- Ezer E., Pálosi E., Hajós G., Rosdy B., Szporny L. Comparative pharmacology of a 1:10 combination of indomethacin-sodium salicylate. Agents Actions. 1979 Apr;9(1):117–123. doi: 10.1007/BF02024142. [DOI] [PubMed] [Google Scholar]
- Gana T. J., Huhlewych R., Koo J. Focal gastric mucosal blood flow in aspirin-induced ulceration. Ann Surg. 1987 Apr;205(4):399–403. doi: 10.1097/00000658-198704000-00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gionchetti P., Guarnieri C., Campieri M., Belluzzi A., Brignola C., Iannone P., Miglioli M., Barbara L. Scavenger effect of sulfasalazine, 5-aminosalicylic acid, and olsalazine on superoxide radical generation. Dig Dis Sci. 1991 Feb;36(2):174–178. doi: 10.1007/BF01300752. [DOI] [PubMed] [Google Scholar]
- Graham D. Y., Smith J. L., Spjut H. J., Torres E. Gastric adaptation. Studies in humans during continuous aspirin administration. Gastroenterology. 1988 Aug;95(2):327–333. [PubMed] [Google Scholar]
- Hawkey C. J., Hawthorne A. B., Hudson N., Cole A. T., Mahida Y. R., Daneshmend T. K. Separation of the impairment of haemostasis by aspirin from mucosal injury in the human stomach. Clin Sci (Lond) 1991 Oct;81(4):565–573. doi: 10.1042/cs0810565. [DOI] [PubMed] [Google Scholar]
- Hawkey C. J. Review article: aspirin and gastrointestinal bleeding. Aliment Pharmacol Ther. 1994 Apr;8(2):141–146. doi: 10.1111/j.1365-2036.1994.tb00271.x. [DOI] [PubMed] [Google Scholar]
- Hayden L. J., Thomas G., West G. B. Inhibitors of gastric lesions in the rat. J Pharm Pharmacol. 1978 Apr;30(4):244–246. doi: 10.1111/j.2042-7158.1978.tb13214.x. [DOI] [PubMed] [Google Scholar]
- Kitahora T., Guth P. H. Effect of aspirin plus hydrochloric acid on the gastric mucosal microcirculation. Gastroenterology. 1987 Oct;93(4):810–817. doi: 10.1016/0016-5085(87)90444-6. [DOI] [PubMed] [Google Scholar]
- Konturek S. J., Kwiecien N., Obtulowicz W., Kiec-Dembinska A., Polanski M., Kopp B., Sito E., Oleksy J. Effect of carprofen and indomethacin on gastric function, mucosal integrity and generation of prostaglandins in men. Hepatogastroenterology. 1982 Dec;29(6):267–270. [PubMed] [Google Scholar]
- Lanza F. L., Royer G. L., Jr, Nelson R. S. Endoscopic evaluation of the effects of aspirin, buffered aspirin, and enteric-coated aspirin on gastric and duodenal mucosa. N Engl J Med. 1980 Jul 17;303(3):136–138. doi: 10.1056/NEJM198007173030305. [DOI] [PubMed] [Google Scholar]
- Ligumsky M., Hansen D. G., Kauffman G. L., Jr Salicylic acid blocks indomethacin- and aspirin-induced cyclo-oxygenase inhibition in rat gastric mucosa. Gastroenterology. 1982 Nov;83(5):1043–1046. [PubMed] [Google Scholar]
- Loh H. S., Watters K., Wilson C. W. The effects of aspirin on the metabolic availability of ascorbic acid in human beings. J Clin Pharmacol. 1973 Nov-Dec;13(11):480–486. doi: 10.1002/j.1552-4604.1973.tb00203.x. [DOI] [PubMed] [Google Scholar]
- Mellors A. J., Nahrwold D. L., Rose R. C. Ascorbic acid flux across mucosal border of guinea pig and human ileum. Am J Physiol. 1977 Nov;233(5):E374–E379. doi: 10.1152/ajpendo.1977.233.5.E374. [DOI] [PubMed] [Google Scholar]
- Neal T. M., Winterbourn C. C., Vissers M. C. Inhibition of neutrophil degranulation and superoxide production by sulfasalazine. Comparison with 5-aminosalicylic acid, sulfapyridine and olsalazine. Biochem Pharmacol. 1987 Sep 1;36(17):2765–2768. doi: 10.1016/0006-2952(87)90262-0. [DOI] [PubMed] [Google Scholar]
- Nielsen O. H., Bouchelouche P. N., Berild D., Ahnfelt-Rønne I. Effect of 5-aminosalicylic acid and analogous substances on superoxide generation and intracellular free calcium in human neutrophilic granulocytes. Scand J Gastroenterol. 1993 Jun;28(6):527–532. doi: 10.3109/00365529309098261. [DOI] [PubMed] [Google Scholar]
- Padh H. Vitamin C: newer insights into its biochemical functions. Nutr Rev. 1991 Mar;49(3):65–70. doi: 10.1111/j.1753-4887.1991.tb07407.x. [DOI] [PubMed] [Google Scholar]
- Perry M. A., Wadhwa S., Parks D. A., Pickard W., Granger D. N. Role of oxygen radicals in ischemia-induced lesions in the cat stomach. Gastroenterology. 1986 Feb;90(2):362–367. doi: 10.1016/0016-5085(86)90933-9. [DOI] [PubMed] [Google Scholar]
- Pihan G., Regillo C., Szabo S. Free radicals and lipid peroxidation in ethanol- or aspirin-induced gastric mucosal injury. Dig Dis Sci. 1987 Dec;32(12):1395–1401. doi: 10.1007/BF01296666. [DOI] [PubMed] [Google Scholar]
- Rachmilewitz D., Karmeli F., Okon E., Samuni A. A novel antiulcerogenic stable radical prevents gastric mucosal lesions in rats. Gut. 1994 Sep;35(9):1181–1188. doi: 10.1136/gut.35.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rathbone B. J., Johnson A. W., Wyatt J. I., Kelleher J., Heatley R. V., Losowsky M. S. Ascorbic acid: a factor concentrated in human gastric juice. Clin Sci (Lond) 1989 Mar;76(3):237–241. doi: 10.1042/cs0760237. [DOI] [PubMed] [Google Scholar]
- Rose R. C., Choi J. L., Koch M. J. Intestinal transport and metabolism of oxidized ascorbic acid (dehydroascorbic acid). Am J Physiol. 1988 Jun;254(6 Pt 1):G824–G828. doi: 10.1152/ajpgi.1988.254.6.G824. [DOI] [PubMed] [Google Scholar]
- Russel R. I., Williamson J. M., Goldberg A., Wares E. Ascorbic-acid levels in leucocytes of patients with gastrointestinal haemorrhage. Lancet. 1968 Sep 14;2(7568):603–606. doi: 10.1016/s0140-6736(68)90698-3. [DOI] [PubMed] [Google Scholar]
- Sahud M. A., Cohen R. J. Effect of aspirin ingestion on ascorbic-acid levels in rheumatoid arthritis. Lancet. 1971 May 8;1(7706):937–938. doi: 10.1016/s0140-6736(71)91441-3. [DOI] [PubMed] [Google Scholar]
- Salim A. S. Gastric mucosal cytoprotection in the rat by scavenging oxygen-derived free radicals. Am J Med Sci. 1991 Nov;302(5):287–291. doi: 10.1097/00000441-199111000-00005. [DOI] [PubMed] [Google Scholar]
- Shorrock C. J., Rees W. D. Mucosal adaptation to indomethacin induced gastric damage in man--studies on morphology, blood flow, and prostaglandin E2 metabolism. Gut. 1992 Feb;33(2):164–169. doi: 10.1136/gut.33.2.164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stevenson N. R. Active transport of L-ascorbic acid in the human ileum. Gastroenterology. 1974 Nov;67(5):952–956. [PubMed] [Google Scholar]
- Taylor T. V., Rimmer S., Day B., Butcher J., Dymock I. W. Ascorbic acid supplementation in the treatment of pressure-sores. Lancet. 1974 Sep 7;2(7880):544–546. doi: 10.1016/s0140-6736(74)91874-1. [DOI] [PubMed] [Google Scholar]
- Torgyán S., Ady E., Wagner L., Neumann T., Béres G., Csányi M. Reduction of indomethacin-induced gastrointestinal blood loss by sodium salicylate in man. Int J Clin Pharmacol Biopharm. 1978 Dec;16(12):610–611. [PubMed] [Google Scholar]
- Vaananen P. M., Meddings J. B., Wallace J. L. Role of oxygen-derived free radicals in indomethacin-induced gastric injury. Am J Physiol. 1991 Sep;261(3 Pt 1):G470–G475. doi: 10.1152/ajpgi.1991.261.3.G470. [DOI] [PubMed] [Google Scholar]
- Wallace J. L., Keenan C. M., Granger D. N. Gastric ulceration induced by nonsteroidal anti-inflammatory drugs is a neutrophil-dependent process. Am J Physiol. 1990 Sep;259(3 Pt 1):G462–G467. doi: 10.1152/ajpgi.1990.259.3.G462. [DOI] [PubMed] [Google Scholar]
- Williams J. G. Phagocytes, toxic oxygen metabolites and inflammatory bowel disease: implications for treatment. Ann R Coll Surg Engl. 1990 Jul;72(4):253–262. [PMC free article] [PubMed] [Google Scholar]
- Yesair D. W., Remington L., Callahan M., Kensler C. J. Comparative effects of salicylic acid, phenylbutazone, probenecid and other anions on the metabolism, distribution and excretion of indomethacin by rats. Biochem Pharmacol. 1970 May;19(5):1591–1600. doi: 10.1016/0006-2952(70)90147-4. [DOI] [PubMed] [Google Scholar]
- Yoshikawa T., Naito Y., Kishi A., Tomii T., Kaneko T., Iinuma S., Ichikawa H., Yasuda M., Takahashi S., Kondo M. Role of active oxygen, lipid peroxidation, and antioxidants in the pathogenesis of gastric mucosal injury induced by indomethacin in rats. Gut. 1993 Jun;34(6):732–737. doi: 10.1136/gut.34.6.732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller J. M., Sullivan B. L. C-reactive protein selectively enhances the intracellular generation of reactive oxygen products by IgG-stimulated monocytes and neutrophils. J Leukoc Biol. 1992 Oct;52(4):449–455. doi: 10.1002/jlb.52.4.449. [DOI] [PubMed] [Google Scholar]


