Abstract
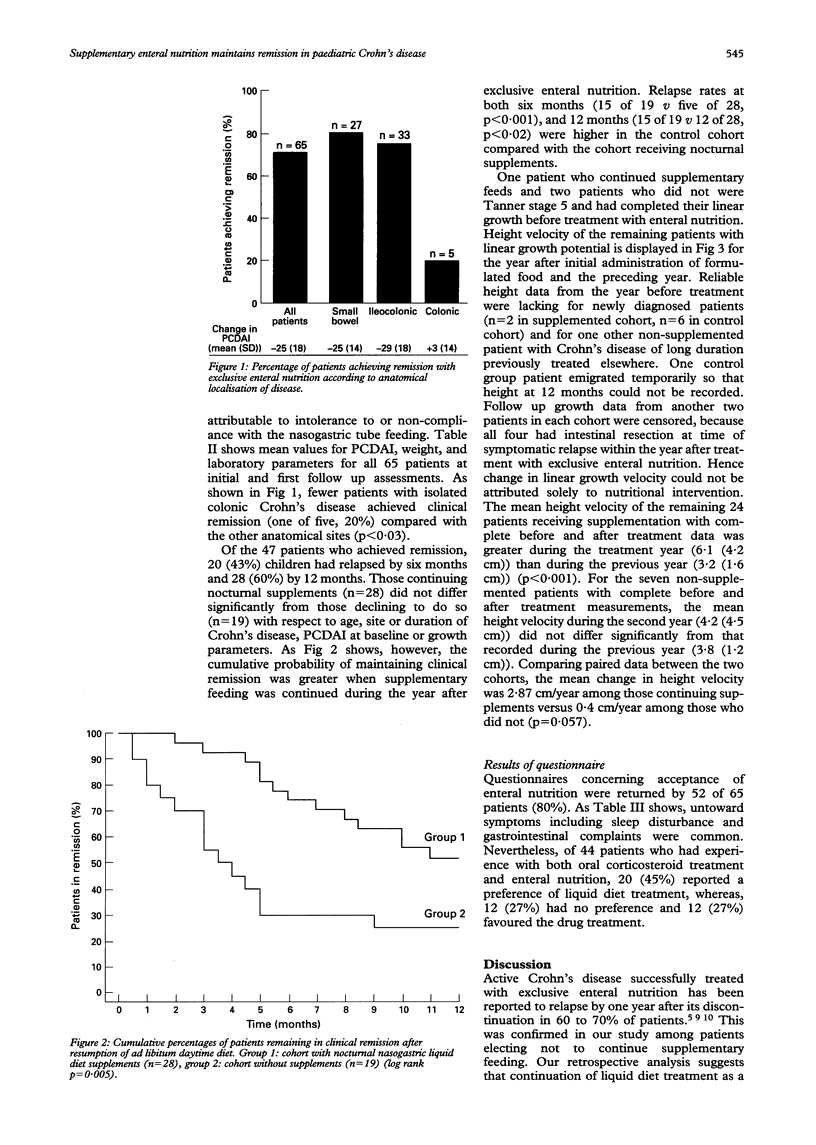
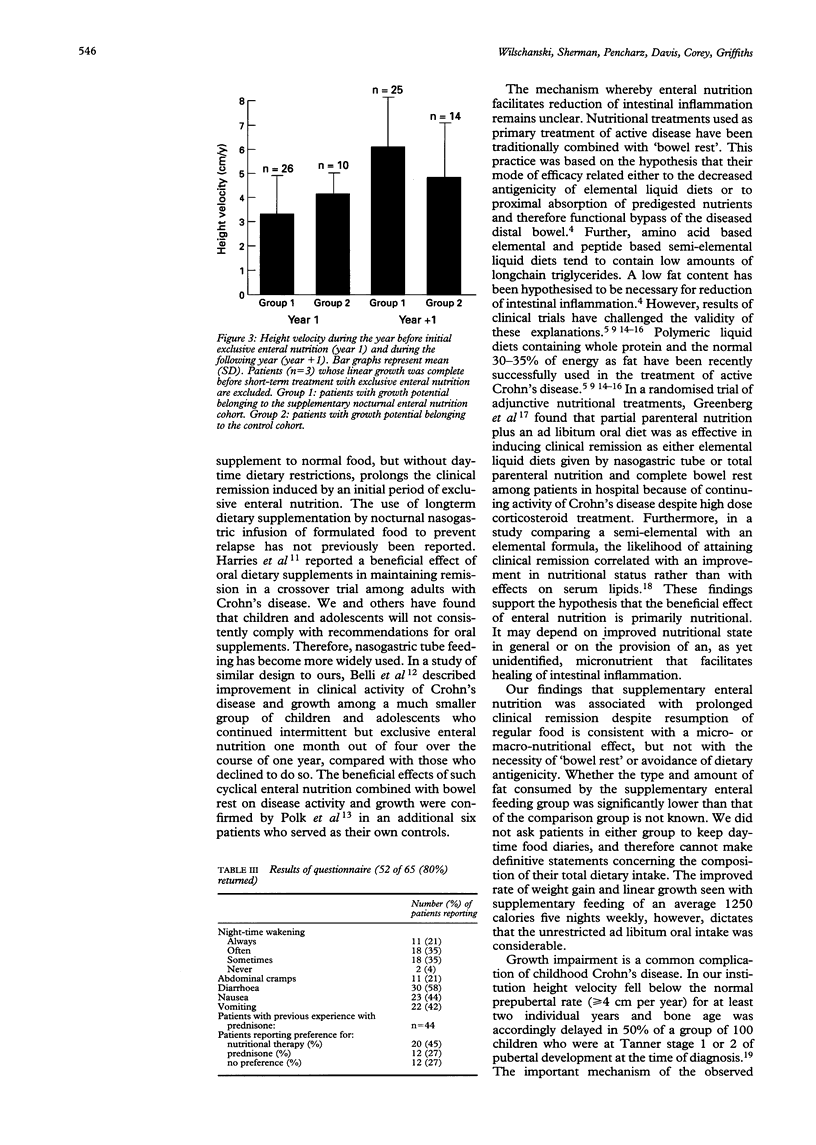
BACKGROUND--Liquid diets given enterally combined with "bowel rest' are efficacious in the treatment of active Crohn's disease, but rapid recrudescence of gastrointestinal symptoms after resumption of a normal diet is common. AIMS--This study examined whether continuation of enteral nutrition as a nocturnal supplement to an ad libitum daytime intake of a normal diet increased the length of remission of Crohn's disease in children. PATIENTS AND METHODS--Children and adolescents with active Crohn's disease treated successfully with exclusive enteral nutrition were classified retrospectively according to whether they continued supplementary enteral nutrition or not. Time to relapse and linear growth were compared between the two cohorts. RESULTS--Between January 1986 and December 1992, 65 patients aged 7-17 years (mean (SD) 13.6 (2.1) years) (36 males, 29 females) with Crohn's disease in exacerbation were treated for > or = four weeks by bowel rest and nasogastric tube feeding of an oligopeptide or amino acid based formula. At first follow up visit, remission (fall in Paediatric Crohn's Disease Activity Index, PCDAI to < or = 20) was achieved in 47 of 65 (72%) patients. Subsequently, 20 of these 47 (43%) relapsed by six months and 28 of 47 (60%) by 12 months. Patients who continued nasogastric supplementary feeding (n = 28) after resumption of an otherwise normal diet remained well longer than those who discontinued nocturnal supplements completely (n = 19) (p < 0.02). Furthermore, continued use of nasogastric supplements before completion of puberty was associated with improved linear growth. CONCLUSION--After successful treatment of active Crohn's disease by exclusive enteral nutrition, supplementary enteral nutrition without restriction of normal diet is associated with prolongation of remission and improved linear growth in children and adolescents.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aiges H., Markowitz J., Rosa J., Daum F. Home nocturnal supplemental nasogastric feedings in growth-retarded adolescents with Crohn's disease. Gastroenterology. 1989 Oct;97(4):905–910. doi: 10.1016/0016-5085(89)91496-0. [DOI] [PubMed] [Google Scholar]
- Belli D. C., Seidman E., Bouthillier L., Weber A. M., Roy C. C., Pletincx M., Beaulieu M., Morin C. L. Chronic intermittent elemental diet improves growth failure in children with Crohn's disease. Gastroenterology. 1988 Mar;94(3):603–610. doi: 10.1016/0016-5085(88)90230-2. [DOI] [PubMed] [Google Scholar]
- Fernández-Bañares F., Cabré E., González-Huix F., Gassull M. A. Enteral nutrition as primary therapy in Crohn's disease. Gut. 1994 Jan;35(1 Suppl):S55–S59. doi: 10.1136/gut.35.1_suppl.s55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- González-Huix F., de León R., Fernández-Bañares F., Esteve M., Cabré E., Acero D., Abad-Lacruz A., Figa M., Guilera M., Planas R. Polymeric enteral diets as primary treatment of active Crohn's disease: a prospective steroid controlled trial. Gut. 1993 Jun;34(6):778–782. doi: 10.1136/gut.34.6.778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorard D. A., Hunt J. B., Payne-James J. J., Palmer K. R., Rees R. G., Clark M. L., Farthing M. J., Misiewicz J. J., Silk D. B. Initial response and subsequent course of Crohn's disease treated with elemental diet or prednisolone. Gut. 1993 Sep;34(9):1198–1202. doi: 10.1136/gut.34.9.1198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenberg G. R., Fleming C. R., Jeejeebhoy K. N., Rosenberg I. H., Sales D., Tremaine W. J. Controlled trial of bowel rest and nutritional support in the management of Crohn's disease. Gut. 1988 Oct;29(10):1309–1315. doi: 10.1136/gut.29.10.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths A. M., Drobnies A., Soldin S. J., Hamilton J. R. Enteric protein loss measured by fecal alpha 1-antitrypsin clearance in the assessment of Crohn's disease activity: a study of children and adolescents. J Pediatr Gastroenterol Nutr. 1986 Nov-Dec;5(6):907–911. doi: 10.1097/00005176-198611000-00015. [DOI] [PubMed] [Google Scholar]
- Griffiths A. M., Nguyen P., Smith C., MacMillan J. H., Sherman P. M. Growth and clinical course of children with Crohn's disease. Gut. 1993 Jul;34(7):939–943. doi: 10.1136/gut.34.7.939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffiths A. M., Ohlsson A., Sherman P. M., Sutherland L. R. Meta-analysis of enteral nutrition as a primary treatment of active Crohn's disease. Gastroenterology. 1995 Apr;108(4):1056–1067. doi: 10.1016/0016-5085(95)90203-1. [DOI] [PubMed] [Google Scholar]
- Harries A. D., Jones L. A., Danis V., Fifield R., Heatley R. V., Newcombe R. G., Rhodes J. Controlled trial of supplemented oral nutrition in Crohn's disease. Lancet. 1983 Apr 23;1(8330):887–890. doi: 10.1016/s0140-6736(83)91325-9. [DOI] [PubMed] [Google Scholar]
- Hyams J. S., Ferry G. D., Mandel F. S., Gryboski J. D., Kibort P. M., Kirschner B. S., Griffiths A. M., Katz A. J., Grand R. J., Boyle J. T. Development and validation of a pediatric Crohn's disease activity index. J Pediatr Gastroenterol Nutr. 1991 May;12(4):439–447. [PubMed] [Google Scholar]
- Kelts D. G., Grand R. J., Shen G., Watkins J. B., Werlin S. L., Boehme C. Nutritional basis of growth failure in children and adolescents with Crohn's disease. Gastroenterology. 1979 Apr;76(4):720–727. [PubMed] [Google Scholar]
- Kirschner B. S., Klich J. R., Kalman S. S., deFavaro M. V., Rosenberg I. H. Reversal of growth retardation in Crohn's disease with therapy emphasizing oral nutritional restitution. Gastroenterology. 1981 Jan;80(1):10–15. [PubMed] [Google Scholar]
- Lindor K. D., Fleming C. R., Burnes J. U., Nelson J. K., Ilstrup D. M. A randomized prospective trial comparing a defined formula diet, corticosteroids, and a defined formula diet plus corticosteroids in active Crohn's disease. Mayo Clin Proc. 1992 Apr;67(4):328–333. doi: 10.1016/s0025-6196(12)61547-x. [DOI] [PubMed] [Google Scholar]
- Lochs H., Steinhardt H. J., Klaus-Wentz B., Zeitz M., Vogelsang H., Sommer H., Fleig W. E., Bauer P., Schirrmeister J., Malchow H. Comparison of enteral nutrition and drug treatment in active Crohn's disease. Results of the European Cooperative Crohn's Disease Study. IV. Gastroenterology. 1991 Oct;101(4):881–888. doi: 10.1016/0016-5085(91)90711-s. [DOI] [PubMed] [Google Scholar]
- Logan R. F., Gillon J., Ferrington C., Ferguson A. Reduction of gastrointestinal protein loss by elemental diet in Crohn's disease of the small bowel. Gut. 1981 May;22(5):383–387. doi: 10.1136/gut.22.5.383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malchow H., Ewe K., Brandes J. W., Goebell H., Ehms H., Sommer H., Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984 Feb;86(2):249–266. [PubMed] [Google Scholar]
- Malchow H., Ewe K., Brandes J. W., Goebell H., Ehms H., Sommer H., Jesdinsky H. European Cooperative Crohn's Disease Study (ECCDS): results of drug treatment. Gastroenterology. 1984 Feb;86(2):249–266. [PubMed] [Google Scholar]
- Motil K. J., Grand R. J., Davis-Kraft L., Ferlic L. L., Smith E. O. Growth failure in children with inflammatory bowel disease: a prospective study. Gastroenterology. 1993 Sep;105(3):681–691. doi: 10.1016/0016-5085(93)90883-e. [DOI] [PubMed] [Google Scholar]
- O'Moráin C., Segal A. W., Levi A. J. Elemental diet as primary treatment of acute Crohn's disease: a controlled trial. Br Med J (Clin Res Ed) 1984 Jun 23;288(6434):1859–1862. doi: 10.1136/bmj.288.6434.1859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polk D. B., Hattner J. A., Kerner J. A., Jr Improved growth and disease activity after intermittent administration of a defined formula diet in children with Crohn's disease. JPEN J Parenter Enteral Nutr. 1992 Nov-Dec;16(6):499–504. doi: 10.1177/0148607192016006499. [DOI] [PubMed] [Google Scholar]
- Raouf A. H., Hildrey V., Daniel J., Walker R. J., Krasner N., Elias E., Rhodes J. M. Enteral feeding as sole treatment for Crohn's disease: controlled trial of whole protein v amino acid based feed and a case study of dietary challenge. Gut. 1991 Jun;32(6):702–707. doi: 10.1136/gut.32.6.702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigaud D., Cosnes J., Le Quintrec Y., René E., Gendre J. P., Mignon M. Controlled trial comparing two types of enteral nutrition in treatment of active Crohn's disease: elemental versus polymeric diet. Gut. 1991 Dec;32(12):1492–1497. doi: 10.1136/gut.32.12.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Royall D., Jeejeebhoy K. N., Baker J. P., Allard J. P., Habal F. M., Cunnane S. C., Greenberg G. R. Comparison of amino acid v peptide based enteral diets in active Crohn's disease: clinical and nutritional outcome. Gut. 1994 Jun;35(6):783–787. doi: 10.1136/gut.35.6.783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruuska T., Savilahti E., Mäki M., Ormälä T., Visakorpi J. K. Exclusive whole protein enteral diet versus prednisolone in the treatment of acute Crohn's disease in children. J Pediatr Gastroenterol Nutr. 1994 Aug;19(2):175–180. doi: 10.1097/00005176-199408000-00006. [DOI] [PubMed] [Google Scholar]
- Sanderson I. R., Boulton P., Menzies I., Walker-Smith J. A. Improvement of abnormal lactulose/rhamnose permeability in active Crohn's disease of the small bowel by an elemental diet. Gut. 1987 Sep;28(9):1073–1076. doi: 10.1136/gut.28.9.1073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidman E., LeLeiko N., Ament M., Berman W., Caplan D., Evans J., Kocoshis S., Lake A., Motil K., Sutphen J. Nutritional issues in pediatric inflammatory bowel disease. J Pediatr Gastroenterol Nutr. 1991 May;12(4):424–438. doi: 10.1097/00005176-199105000-00004. [DOI] [PubMed] [Google Scholar]
- Singleton J. W., Hanauer S. B., Gitnick G. L., Peppercorn M. A., Robinson M. G., Wruble L. D., Krawitt E. L. Mesalamine capsules for the treatment of active Crohn's disease: results of a 16-week trial. Pentasa Crohn's Disease Study Group. Gastroenterology. 1993 May;104(5):1293–1301. doi: 10.1016/0016-5085(93)90337-c. [DOI] [PubMed] [Google Scholar]
- Summers R. W., Switz D. M., Sessions J. T., Jr, Becktel J. M., Best W. R., Kern F., Jr, Singleton J. W. National Cooperative Crohn's Disease Study: results of drug treatment. Gastroenterology. 1979 Oct;77(4 Pt 2):847–869. [PubMed] [Google Scholar]
- Teahon K., Bjarnason I., Pearson M., Levi A. J. Ten years' experience with an elemental diet in the management of Crohn's disease. Gut. 1990 Oct;31(10):1133–1137. doi: 10.1136/gut.31.10.1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teahon K., Smethurst P., Pearson M., Levi A. J., Bjarnason I. The effect of elemental diet on intestinal permeability and inflammation in Crohn's disease. Gastroenterology. 1991 Jul;101(1):84–89. doi: 10.1016/0016-5085(91)90463-u. [DOI] [PubMed] [Google Scholar]


