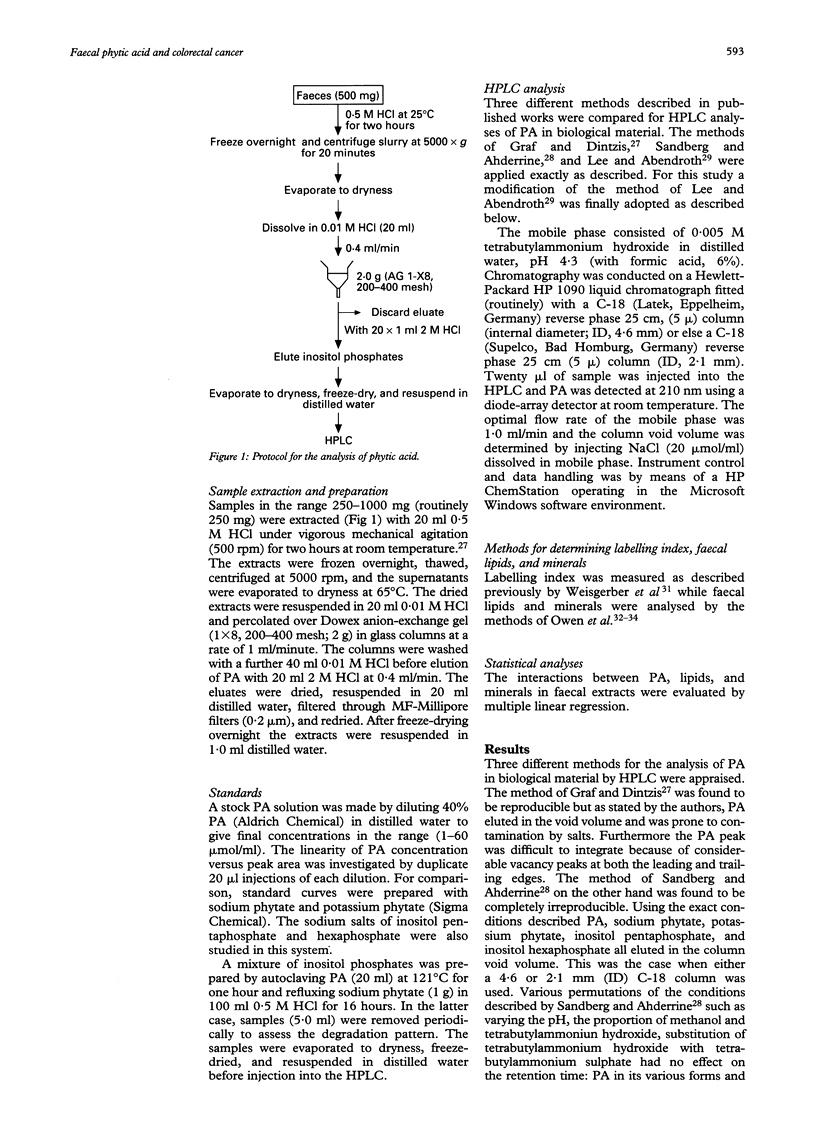
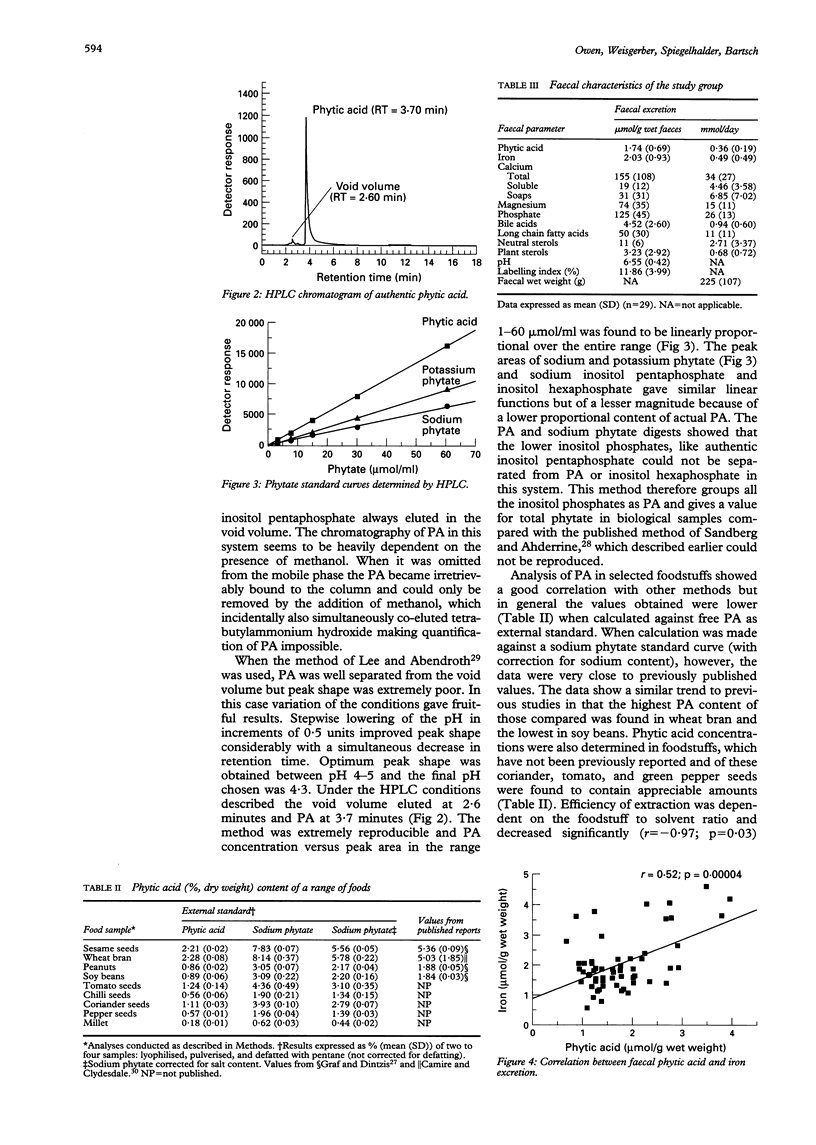
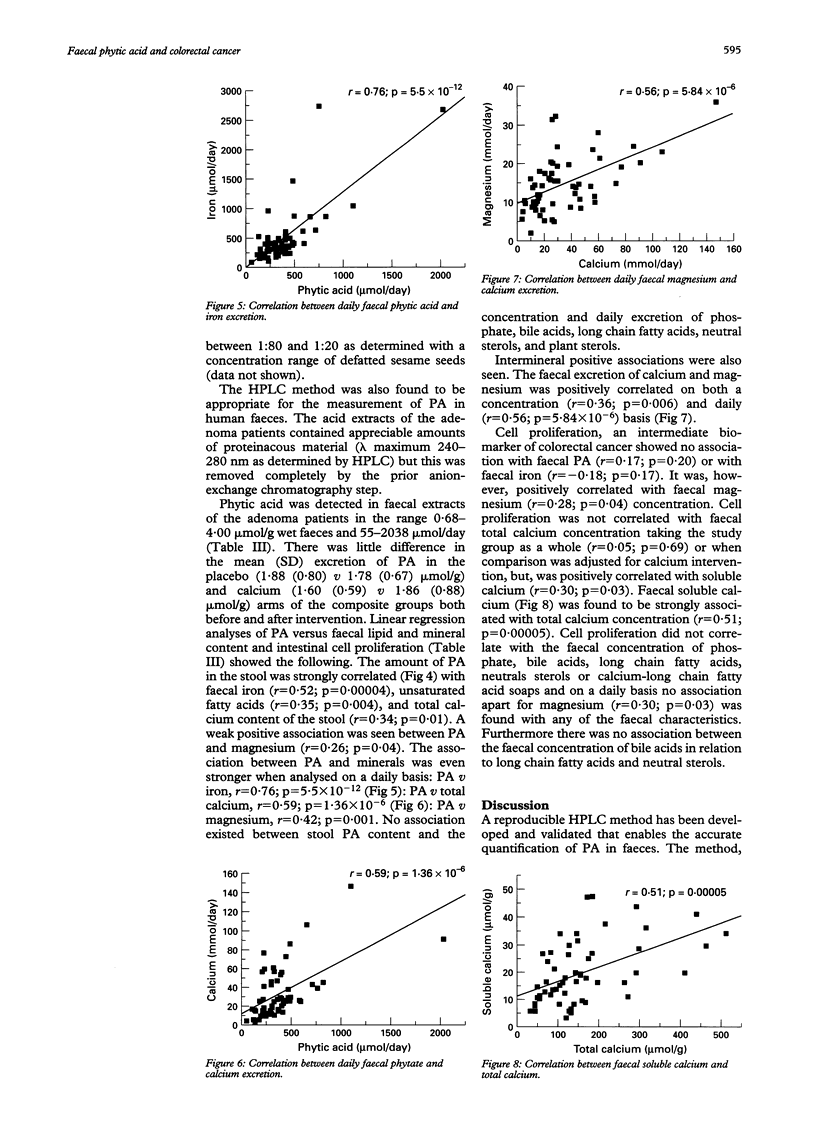
Abstract
AIMS--Phytic acid, a major constituent of cereals, pulses, and seeds has been advocated as an important antioxidant component of dietary fibre that affords possible protection against colorectal cancer. This is supported by experimental studies showing it has antineoplastic activity in animal models of both colon and breast cancer. To date the concentration of faecal phytic acid in human clinical groups has not been evaluated. Therefore the faecal phytic acid content of adenoma patients drawn from a placebo controlled calcium intervention trial was evaluated. METHODS--Phytic acid was measured in faecal extracts by an improved ion-pair high performance liquid chromatography method. RESULTS--Phytic acid was detected in the range 0.68-4.00 mumol/g wet faeces and 55-2038 mumol/day. Linear regression analyses showed no association between stool phytic acid and lipid content. Strong correlations were seen, however, between phytic acid and iron content, both on a concentration (r = 0.52; p = 0.00004) and daily excretion (r = 0.76; p = 5.5 x 10(-12) basis. Phytic acid was also strongly correlated with the daily excretion of calcium (r = 0.59; p = 1.36 x 10(-6) and magnesium (r = 0.42; p = 0.001). Cell proliferation in the sigmoid colon, an intermediate biomarker of colorectal cancer was not significantly associated with faecal phytic acid, minerals or lipid content in this compromised clinical group. CONCLUSIONS--This improved method, developed for the determination of phytic acid in faeces should allow further studies on the role of phytic acid in the aetiology of colorectal cancer to be conducted on a population or case control basis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Armstrong B., Doll R. Environmental factors and cancer incidence and mortality in different countries, with special reference to dietary practices. Int J Cancer. 1975 Apr 15;15(4):617–631. doi: 10.1002/ijc.2910150411. [DOI] [PubMed] [Google Scholar]
- Burkitt D. P. Epidemiology of cancer of the colon and rectum. Cancer. 1971 Jul;28(1):3–13. doi: 10.1002/1097-0142(197107)28:1<3::aid-cncr2820280104>3.0.co;2-n. [DOI] [PubMed] [Google Scholar]
- Costa N. M., Low A. G., Walker A. F., Owen R. W., Englyst H. N. Effect of baked beans (Phaseolus vulgaris) on steroid metabolism and non-starch polysaccharide output of hypercholesterolaemic pigs with or without an ileo-rectal anastomosis. Br J Nutr. 1994 Jun;71(6):871–886. doi: 10.1079/bjn19940193. [DOI] [PubMed] [Google Scholar]
- Cummings J. H., Bingham S. A., Heaton K. W., Eastwood M. A. Fecal weight, colon cancer risk, and dietary intake of nonstarch polysaccharides (dietary fiber) Gastroenterology. 1992 Dec;103(6):1783–1789. doi: 10.1016/0016-5085(92)91435-7. [DOI] [PubMed] [Google Scholar]
- Fadden K., Owen R. W. Faecal steroids and colorectal cancer: the effect of lactulose on faecal bacterial metabolism in a continuous culture model of the large intestine. Eur J Cancer Prev. 1992 Feb;1(2):113–127. [PubMed] [Google Scholar]
- Giovannucci E., Rimm E. B., Stampfer M. J., Colditz G. A., Ascherio A., Willett W. C. Intake of fat, meat, and fiber in relation to risk of colon cancer in men. Cancer Res. 1994 May 1;54(9):2390–2397. [PubMed] [Google Scholar]
- Graf E., Dintzis F. R. High-performance liquid chromatographic method for the determination of phytate. Anal Biochem. 1982 Jan 15;119(2):413–417. doi: 10.1016/0003-2697(82)90606-6. [DOI] [PubMed] [Google Scholar]
- Graf E., Eaton J. W. Antioxidant functions of phytic acid. Free Radic Biol Med. 1990;8(1):61–69. doi: 10.1016/0891-5849(90)90146-a. [DOI] [PubMed] [Google Scholar]
- Graf E., Eaton J. W. Dietary suppression of colonic cancer. Fiber or phytate? Cancer. 1985 Aug 15;56(4):717–718. doi: 10.1002/1097-0142(19850815)56:4<717::aid-cncr2820560402>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- Graf E., Mahoney J. R., Bryant R. G., Eaton J. W. Iron-catalyzed hydroxyl radical formation. Stringent requirement for free iron coordination site. J Biol Chem. 1984 Mar 25;259(6):3620–3624. [PubMed] [Google Scholar]
- Hill M. J., Drasar B. S., Williams R. E., Meade T. W., Cox A. G., Simpson J. E., Morson B. C. Faecal bile-acids and clostridia in patients with cancer of the large bowel. Lancet. 1975 Mar 8;1(7906):535–539. doi: 10.1016/s0140-6736(75)91556-1. [DOI] [PubMed] [Google Scholar]
- Howe G. R., Benito E., Castelleto R., Cornée J., Estève J., Gallagher R. P., Iscovich J. M., Deng-ao J., Kaaks R., Kune G. A. Dietary intake of fiber and decreased risk of cancers of the colon and rectum: evidence from the combined analysis of 13 case-control studies. J Natl Cancer Inst. 1992 Dec 16;84(24):1887–1896. doi: 10.1093/jnci/84.24.1887. [DOI] [PubMed] [Google Scholar]
- Iqbal T. H., Lewis K. O., Cooper B. T. Phytase activity in the human and rat small intestine. Gut. 1994 Sep;35(9):1233–1236. doi: 10.1136/gut.35.9.1233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson R. L., Davis F. G., Sutter E., Sobin L. H., Kikendall J. W., Bowen P. Body iron stores and risk of colonic neoplasia. J Natl Cancer Inst. 1994 Mar 16;86(6):455–460. doi: 10.1093/jnci/86.6.455. [DOI] [PubMed] [Google Scholar]
- Nelson R. L., Yoo S. J., Tanure J. C., Andrianopoulos G., Misumi A. The effect of iron on experimental colorectal carcinogenesis. Anticancer Res. 1989 Nov-Dec;9(6):1477–1482. [PubMed] [Google Scholar]
- Nielsen B. K., Thompson L. U., Bird R. P. Effect of phytic acid on colonic epithelial cell proliferation. Cancer Lett. 1987 Nov;37(3):317–325. doi: 10.1016/0304-3835(87)90117-0. [DOI] [PubMed] [Google Scholar]
- Ohlrogge J. B., Kernan T. P. Oxygen-dependent aging of seeds. Plant Physiol. 1982 Sep;70(3):791–794. doi: 10.1104/pp.70.3.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen R. W., Thompson M. H., Hill M. J. Analysis of metabolic profiles of steroids in faeces of healthy subjects undergoing chenodeoxycholic acid treatment by liquid-gel chromatography and gas-liquid chromatography-mass spectrometry. J Steroid Biochem. 1984 Nov;21(5):593–600. doi: 10.1016/0022-4731(84)90336-4. [DOI] [PubMed] [Google Scholar]
- Owen R. W., Weisgerber U. M., Carr J., Harrison M. H. Analysis of calcium-lipid complexes in faeces. Eur J Cancer Prev. 1995 Jun;4(3):247–255. doi: 10.1097/00008469-199506000-00006. [DOI] [PubMed] [Google Scholar]
- Reddy B. S., Wynder E. L. Metabolic epidemiology of colon cancer. Fecal bile acids and neutral sterols in colon cancer patients and patients with adenomatous polyps. Cancer. 1977 Jun;39(6):2533–2539. doi: 10.1002/1097-0142(197706)39:6<2533::aid-cncr2820390634>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Risio M., Lipkin M., Candelaresi G., Bertone A., Coverlizza S., Rossini F. P. Correlations between rectal mucosa cell proliferation and the clinical and pathological features of nonfamilial neoplasia of the large intestine. Cancer Res. 1991 Apr 1;51(7):1917–1921. [PubMed] [Google Scholar]
- Shamsuddin A. M., Elsayed A. M., Ullah A. Suppression of large intestinal cancer in F344 rats by inositol hexaphosphate. Carcinogenesis. 1988 Apr;9(4):577–580. doi: 10.1093/carcin/9.4.577. [DOI] [PubMed] [Google Scholar]
- Shamsuddin A. M., Ullah A. Inositol hexaphosphate inhibits large intestinal cancer in F344 rats 5 months after induction by azoxymethane. Carcinogenesis. 1989 Mar;10(3):625–626. doi: 10.1093/carcin/10.3.625. [DOI] [PubMed] [Google Scholar]
- Siegers C. P., Bumann D., Baretton G., Younes M. Dietary iron enhances the tumor rate in dimethylhydrazine-induced colon carcinogenesis in mice. Cancer Lett. 1988 Aug 30;41(3):251–256. doi: 10.1016/0304-3835(88)90285-6. [DOI] [PubMed] [Google Scholar]
- Siegers C. P., Bumann D., Trepkau H. D., Schadwinkel B., Baretton G. Influence of dietary iron overload on cell proliferation and intestinal tumorigenesis in mice. Cancer Lett. 1992 Aug 31;65(3):245–249. doi: 10.1016/0304-3835(92)90239-r. [DOI] [PubMed] [Google Scholar]
- Stevens R. G., Jones D. Y., Micozzi M. S., Taylor P. R. Body iron stores and the risk of cancer. N Engl J Med. 1988 Oct 20;319(16):1047–1052. doi: 10.1056/NEJM198810203191603. [DOI] [PubMed] [Google Scholar]
- Thompson L. U., Zhang L. Phytic acid and minerals: effect on early markers of risk for mammary and colon carcinogenesis. Carcinogenesis. 1991 Nov;12(11):2041–2045. doi: 10.1093/carcin/12.11.2041. [DOI] [PubMed] [Google Scholar]
- Torre M., Rodriguez A. R., Saura-Calixto F. Effects of dietary fiber and phytic acid on mineral availability. Crit Rev Food Sci Nutr. 1991;30(1):1–22. doi: 10.1080/10408399109527539. [DOI] [PubMed] [Google Scholar]
- Ullah A., Shamsuddin A. M. Dose-dependent inhibition of large intestinal cancer by inositol hexaphosphate in F344 rats. Carcinogenesis. 1990 Dec;11(12):2219–2222. doi: 10.1093/carcin/11.12.2219. [DOI] [PubMed] [Google Scholar]
- Weisgerber U. M., Boeing H., Nemitz R., Raedsch R., Waldherr R. Proliferation cell nuclear antigen (clone 19A2) correlates with 5-bromo-2-deoxyuridine labelling in human colonic epithelium. Gut. 1993 Nov;34(11):1587–1592. doi: 10.1136/gut.34.11.1587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett W. C., Stampfer M. J., Colditz G. A., Rosner B. A., Speizer F. E. Relation of meat, fat, and fiber intake to the risk of colon cancer in a prospective study among women. N Engl J Med. 1990 Dec 13;323(24):1664–1672. doi: 10.1056/NEJM199012133232404. [DOI] [PubMed] [Google Scholar]



