Abstract
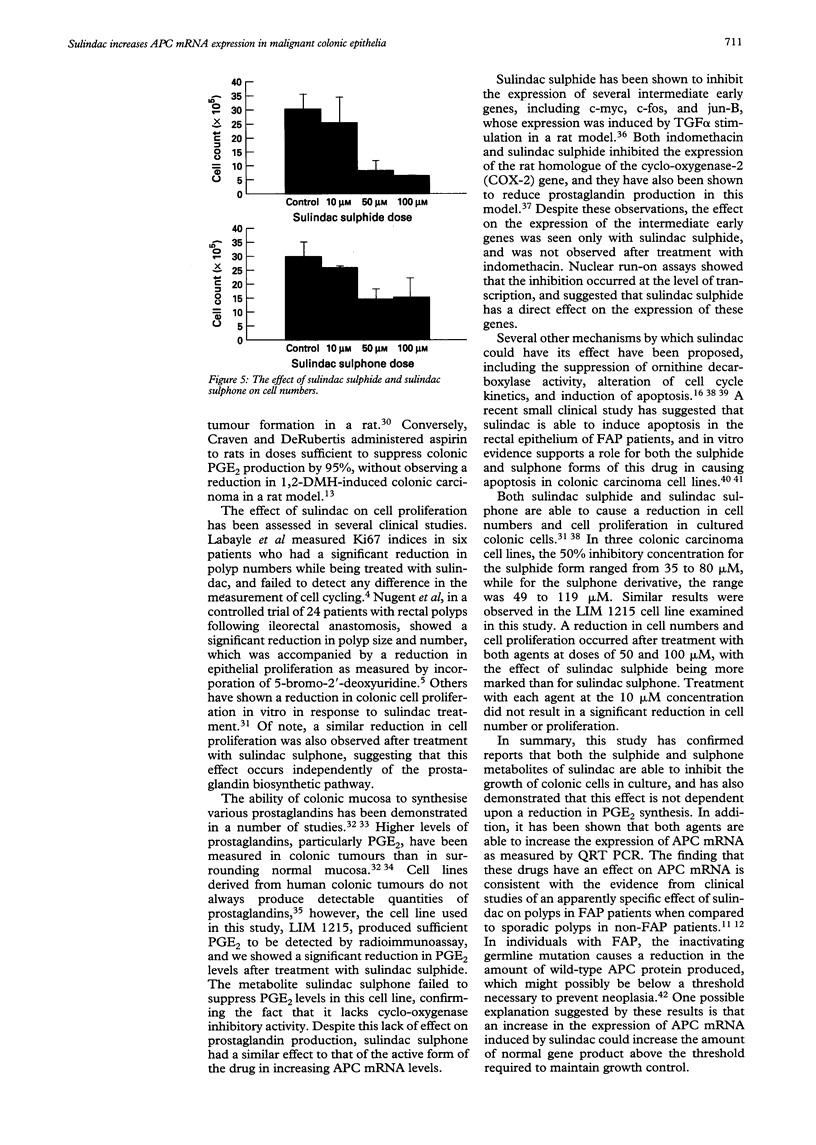
BACKGROUND--Sulindac is a non-steroidal anti-inflammatory drug which induces regression of colonic polyps in patients with familial adenomatous polyposis. Animal and in vitro studies have shown that both the sulphide metabolite of sulindac, which is able to inhibit cyclo-oxygenase, and the sulphone metabolite, which lacks this ability, are able to inhibit the growth of colonic carcinoma cells. The exact mechanism by which these effects occurs is not known. AIMS--To examine the effect of sulindac sulphide and sulindac sulphone on the expression of APC messenger RNA (mRNA), and on the proliferation of colonic carcinoma cells in vitro. METHODS--The colonic carcinoma cell line LIM 1215 was treated with sulindac sulphide and sulindac sulphone (10 microM or 100 microM) for 24 hours. Total RNA was extracted and APC mRNA was quantitated using competitive reverse transcription polymerase chain reaction. Measurements of cell number, cell proliferation, and prostaglandin E2 concentrations were also made. RESULTS--A significant increase in APC mRNA was observed after treatment with 10 microM of both sulindac sulphide and sulindac sulphone (control: 37.2 (19.7); 10 microM sulindac sulphide: 129 (112.8); 10 microM sulindac sulphone: 207.7 (102.9) pg/(g total RNA) (p < 0.05). Prostaglandin E2 concentrations were significantly reduced after treatment with sulindac sulphide, but not after sulindac sulphone. Both agents produced a dose dependent reduction in cell numbers and cell proliferation, which was more noticeable after treatment with sulindac sulphide. CONCLUSIONS--Both sulindac sulphide and sulindac sulphone inhibit the growth of carcinoma cells in vitro and cause an increase in APC mRNA. The effect of these agents on colonic carcinogenesis is not mediated entirely by means of an inhibition of prostaglandin biosynthesis.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bennett A., Tacca M. D., Stamford I. F., Zebro T. Prostaglandins from tumours of human large bowel. Br J Cancer. 1977 Jun;35(6):881–884. doi: 10.1038/bjc.1977.132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodmer W. F., Bailey C. J., Bodmer J., Bussey H. J., Ellis A., Gorman P., Lucibello F. C., Murday V. A., Rider S. H., Scambler P. Localization of the gene for familial adenomatous polyposis on chromosome 5. Nature. 1987 Aug 13;328(6131):614–616. doi: 10.1038/328614a0. [DOI] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Craven P. A., DeRubertis F. R. Effects of aspirin on 1,2-dimethylhydrazine-induced colonic carcinogenesis. Carcinogenesis. 1992 Apr;13(4):541–546. doi: 10.1093/carcin/13.4.541. [DOI] [PubMed] [Google Scholar]
- Craven P. A., Thornburg K., DeRubertis F. R. Sustained increase in the proliferation of rat colonic mucosa during chronic treatment with aspirin. Gastroenterology. 1988 Mar;94(3):567–575. doi: 10.1016/0016-5085(88)90225-9. [DOI] [PubMed] [Google Scholar]
- Debinski H. S., Trojan J., Nugent K. P., Spigelman A. D., Phillips R. K. Effect of sulindac on small polyps in familial adenomatous polyposis. Lancet. 1995 Apr 1;345(8953):855–856. [PubMed] [Google Scholar]
- DuBois R. N., Awad J., Morrow J., Roberts L. J., 2nd, Bishop P. R. Regulation of eicosanoid production and mitogenesis in rat intestinal epithelial cells by transforming growth factor-alpha and phorbol ester. J Clin Invest. 1994 Feb;93(2):493–498. doi: 10.1172/JCI116998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duggan D. E., Hooke K. F., Risley E. A., Shen T. Y., Arman C. G. Identification of the biologically active form of sulindac. J Pharmacol Exp Ther. 1977 Apr;201(1):8–13. [PubMed] [Google Scholar]
- Giardiello F. M., Hamilton S. R., Krush A. J., Piantadosi S., Hylind L. M., Celano P., Booker S. V., Robinson C. R., Offerhaus G. J. Treatment of colonic and rectal adenomas with sulindac in familial adenomatous polyposis. N Engl J Med. 1993 May 6;328(18):1313–1316. doi: 10.1056/NEJM199305063281805. [DOI] [PubMed] [Google Scholar]
- Groden J., Thliveris A., Samowitz W., Carlson M., Gelbert L., Albertsen H., Joslyn G., Stevens J., Spirio L., Robertson M. Identification and characterization of the familial adenomatous polyposis coli gene. Cell. 1991 Aug 9;66(3):589–600. doi: 10.1016/0092-8674(81)90021-0. [DOI] [PubMed] [Google Scholar]
- Hixson L. J., Alberts D. S., Krutzsch M., Einsphar J., Brendel K., Gross P. H., Paranka N. S., Baier M., Emerson S., Pamukcu R. Antiproliferative effect of nonsteroidal antiinflammatory drugs against human colon cancer cells. Cancer Epidemiol Biomarkers Prev. 1994 Jul-Aug;3(5):433–438. [PubMed] [Google Scholar]
- Hixson L. J., Earnest D. L., Fennerty M. B., Sampliner R. E. NSAID effect on sporadic colon polyps. Am J Gastroenterol. 1993 Oct;88(10):1652–1656. [PubMed] [Google Scholar]
- Hubbard W. C., Alley M. C., McLemore T. L., Boyd M. R. Profiles of prostaglandin biosynthesis in sixteen established cell lines derived from human lung, colon, prostate, and ovarian tumors. Cancer Res. 1988 Sep 1;48(17):4770–4775. [PubMed] [Google Scholar]
- Hucker H. B., Stauffer S. C., White S. D., Rhodes R. E., Arison B. H., Umbenhauer E. R., Bower R. J., McMahon F. G. Physiologic disposition and metabolic fate of a new anti-inflammatory agent, cis-5-fluro-2-methyl-1-(p-(methylsulfinyl)-benzylidenyl)-indene-3-acetic acid in the rat, dog, rhesus monkey, and man. Drug Metab Dispos. 1973 Nov-Dec;1(6):721–736. [PubMed] [Google Scholar]
- Kinzler K. W., Nilbert M. C., Su L. K., Vogelstein B., Bryan T. M., Levy D. B., Smith K. J., Preisinger A. C., Hedge P., McKechnie D. Identification of FAP locus genes from chromosome 5q21. Science. 1991 Aug 9;253(5020):661–665. doi: 10.1126/science.1651562. [DOI] [PubMed] [Google Scholar]
- Knapp H. R., Oelz O., Sweetman B. J., Oates J. A. Synthesis and metabolism of prostaglandins E2, F2alpha and D2 by the rat gastrointestinal tract. Stimulation by a hypertonic environment in vitro. Prostaglandins. 1978 May;15(5):751–757. doi: 10.1016/0090-6980(78)90141-7. [DOI] [PubMed] [Google Scholar]
- Labayle D., Fischer D., Vielh P., Drouhin F., Pariente A., Bories C., Duhamel O., Trousset M., Attali P. Sulindac causes regression of rectal polyps in familial adenomatous polyposis. Gastroenterology. 1991 Sep;101(3):635–639. doi: 10.1016/0016-5085(91)90519-q. [DOI] [PubMed] [Google Scholar]
- Ladenheim J., Garcia G., Titzer D., Herzenberg H., Lavori P., Edson R., Omary M. B. Effect of sulindac on sporadic colonic polyps. Gastroenterology. 1995 Apr;108(4):1083–1087. doi: 10.1016/0016-5085(95)90206-6. [DOI] [PubMed] [Google Scholar]
- Lim S. K., Sigmund C. D., Gross K. W., Maquat L. E. Nonsense codons in human beta-globin mRNA result in the production of mRNA degradation products. Mol Cell Biol. 1992 Mar;12(3):1149–1161. doi: 10.1128/mcb.12.3.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch H. T., Thorson A. G., Smyrk T. Rectal cancer after prolonged sulindac chemoprevention. A case report. Cancer. 1995 Feb 15;75(4):936–938. doi: 10.1002/1097-0142(19950215)75:4<936::aid-cncr2820750407>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Miyoshi Y., Nagase H., Ando H., Horii A., Ichii S., Nakatsuru S., Aoki T., Miki Y., Mori T., Nakamura Y. Somatic mutations of the APC gene in colorectal tumors: mutation cluster region in the APC gene. Hum Mol Genet. 1992 Jul;1(4):229–233. doi: 10.1093/hmg/1.4.229. [DOI] [PubMed] [Google Scholar]
- Munemitsu S., Albert I., Souza B., Rubinfeld B., Polakis P. Regulation of intracellular beta-catenin levels by the adenomatous polyposis coli (APC) tumor-suppressor protein. Proc Natl Acad Sci U S A. 1995 Mar 28;92(7):3046–3050. doi: 10.1073/pnas.92.7.3046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munemitsu S., Souza B., Müller O., Albert I., Rubinfeld B., Polakis P. The APC gene product associates with microtubules in vivo and promotes their assembly in vitro. Cancer Res. 1994 Jul 15;54(14):3676–3681. [PubMed] [Google Scholar]
- Nagase H., Nakamura Y. Mutations of the APC (adenomatous polyposis coli) gene. Hum Mutat. 1993;2(6):425–434. doi: 10.1002/humu.1380020602. [DOI] [PubMed] [Google Scholar]
- Narisawa T., Hermanek P., Habs M., Schmähl D. Reduction of carcinogenicity of N-nitrosomethylurea by indomethacin and failure of resuming effect of prostaglandin E2 (PGE2) against indomethacin. J Cancer Res Clin Oncol. 1984;108(2):239–242. doi: 10.1007/BF00402475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niv Y., Fraser G. M. Adenocarcinoma in the rectal segment in familial polyposis coli is not prevented by sulindac therapy. Gastroenterology. 1994 Sep;107(3):854–857. doi: 10.1016/0016-5085(94)90136-8. [DOI] [PubMed] [Google Scholar]
- Nugent K. P., Farmer K. C., Spigelman A. D., Williams C. B., Phillips R. K. Randomized controlled trial of the effect of sulindac on duodenal and rectal polyposis and cell proliferation in patients with familial adenomatous polyposis. Br J Surg. 1993 Dec;80(12):1618–1619. doi: 10.1002/bjs.1800801244. [DOI] [PubMed] [Google Scholar]
- Pollard M., Luckert P. H. Effect of indomethacin on intestinal tumors induced in rats by the acetate derivative of dimethylnitrosamine. Science. 1981 Oct 30;214(4520):558–559. doi: 10.1126/science.7291992. [DOI] [PubMed] [Google Scholar]
- Pugh S., Thomas G. A. Patients with adenomatous polyps and carcinomas have increased colonic mucosal prostaglandin E2. Gut. 1994 May;35(5):675–678. doi: 10.1136/gut.35.5.675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigau J., Piqué J. M., Rubio E., Planas R., Tarrech J. M., Bordas J. M. Effects of long-term sulindac therapy on colonic polyposis. Ann Intern Med. 1991 Dec 15;115(12):952–954. doi: 10.7326/0003-4819-115-12-952. [DOI] [PubMed] [Google Scholar]
- Rubinfeld B., Souza B., Albert I., Müller O., Chamberlain S. H., Masiarz F. R., Munemitsu S., Polakis P. Association of the APC gene product with beta-catenin. Science. 1993 Dec 10;262(5140):1731–1734. doi: 10.1126/science.8259518. [DOI] [PubMed] [Google Scholar]
- Smith K. J., Johnson K. A., Bryan T. M., Hill D. E., Markowitz S., Willson J. K., Paraskeva C., Petersen G. M., Hamilton S. R., Vogelstein B. The APC gene product in normal and tumor cells. Proc Natl Acad Sci U S A. 1993 Apr 1;90(7):2846–2850. doi: 10.1073/pnas.90.7.2846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith K. J., Levy D. B., Maupin P., Pollard T. D., Vogelstein B., Kinzler K. W. Wild-type but not mutant APC associates with the microtubule cytoskeleton. Cancer Res. 1994 Jul 15;54(14):3672–3675. [PubMed] [Google Scholar]
- Su L. K., Johnson K. A., Smith K. J., Hill D. E., Vogelstein B., Kinzler K. W. Association between wild type and mutant APC gene products. Cancer Res. 1993 Jun 15;53(12):2728–2731. [PubMed] [Google Scholar]
- Su L. K., Vogelstein B., Kinzler K. W. Association of the APC tumor suppressor protein with catenins. Science. 1993 Dec 10;262(5140):1734–1737. doi: 10.1126/science.8259519. [DOI] [PubMed] [Google Scholar]
- Verma A. K., Ashendel C. L., Boutwell R. K. Inhibition by prostaglandin synthesis inhibitors of the induction of epidermal ornithine decarboxylase activity, the accumulation of prostaglandins, and tumor promotion caused by 12-O-tetradecanoylphorbol-13-acetate. Cancer Res. 1980 Feb;40(2):308–315. [PubMed] [Google Scholar]
- Waddell W. R., Ganser G. F., Cerise E. J., Loughry R. W. Sulindac for polyposis of the colon. Am J Surg. 1989 Jan;157(1):175–179. doi: 10.1016/0002-9610(89)90442-x. [DOI] [PubMed] [Google Scholar]
- Waddell W. R., Loughry R. W. Sulindac for polyposis of the colon. J Surg Oncol. 1983 Sep;24(1):83–87. doi: 10.1002/jso.2930240119. [DOI] [PubMed] [Google Scholar]
- Whitehead R. H., Macrae F. A., St John D. J., Ma J. A colon cancer cell line (LIM1215) derived from a patient with inherited nonpolyposis colorectal cancer. J Natl Cancer Inst. 1985 Apr;74(4):759–765. [PubMed] [Google Scholar]




